Today’s post is a guest blog from Michael Curtin in the cellular analysis and proteomics group at Promega.
Glycobiology is the study of carbohydrates and their role in biology. Glycans, defined as “compounds consisting of a large number of monosaccharides linked glycosidically” are present in all living cells and coat cell membranes and are integral components of cell walls (1). They play diverse roles, including critical functions in cell signaling, molecular recognition, immunity and inflammation. They are the cell-surface molecules that define the ABO blood groups and must be taken into consideration to ensure successful blood transfusions. (2).The process by which a sugar moiety is attached to a biological compound is referred to as glycosylation. Protein glycosylation is a form of post-translational modification, which is important for many biological processes and often serves as an analog switch that modulates protein activity.The class of enzymes responsible for transferring the sugar moiety onto proteins is called a glycosyltransferase (GT).
GTs can be divided into three major types based on their roles:
- Oligosaccharide elongation for peptidoglycan biosynthesis
- Regulation of protein activities by post-translational modification
- Small molecule glucuronidation as means of drug metabolism
Examples of GTs include:
- Arabinosyltransferase
- Galactosyltransferase
- Glucosyltransferase
- O-GlcNac-transferase
- GalNac-transferase
- Fructosyltransferase
- Glucosylceramide synthase
- Galactosylceramide synthase
The nomenclature for GTs is based on the type of sugar the enzymes transfer to a target molecule (e.g., galactosyltransferases transfer galactose). However, glycosyltransferases cannot simply transfer a monosaccharide by itself—the monosacharide must be conjugated to a nucleotide. The most common nucleotide used by glycosyltransferases is uridine diphosphate (UDP). Other nucleotides involved in glycosylation reactions include guanosine diphosphate (GDP) and cytidine monophosphate (CMP)-silaic acid.
GTs are widely studied, and because of their implication in disease states, some are potential drug targets. The following passage was taken direct from the 2012 report from the National Research Council of the National Academies “Transforming glycoscience: A Roadmap to the Future” (2):
In human health, glycans are involved in myriad processes that are part of normal physiology, development, and cell signaling, along with the development of both chronic and infectious diseases. For example, glycans on cell surfaces are important in molecular recognition. One example of this function is their role in the movement of white blood cells through the body to a site of infection, enabling the immune system to respond where needed. Much of the information content in cells is encompassed in the glycome. Glycans contain key biological information that complements the information stored in DNA to help complete the link between genotype and phenotype or between the genome and expressed traits. Many advances in understanding human health and diseases are the result of current knowledge about nucleic acids, proteins, and glycans and how these vary in different circumstances and in different people. However, much is still unknown. Continued advances in understanding the biological roles played by glycans, along with the factors that influence or alter their functions, will have consequences for the fundamental understanding of biology and will contribute to the development of new therapeutic medicines.
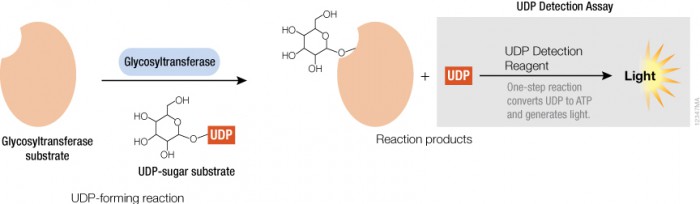 Thus, assays that measure glycosyltransferase activities are desirable to study and understand their mode of regulation and to identify selective and potent inhibitors as potential therapeutics. To this end, Promega is developing assays to address the needs of the glycosylation community—starting with a bioluminescent assay to detect UDP, the key product of the glycosyltransferase reaction. Following the glycosyltransferase reaction the assay converts UDP to ATP, which can then be used in a coupled reaction with Ultra-Glo™ Luciferase to produce light (see figure). This assay will help researchers learn more about protein glycosylation and help speed up the development of future therapeutics.
Thus, assays that measure glycosyltransferase activities are desirable to study and understand their mode of regulation and to identify selective and potent inhibitors as potential therapeutics. To this end, Promega is developing assays to address the needs of the glycosylation community—starting with a bioluminescent assay to detect UDP, the key product of the glycosyltransferase reaction. Following the glycosyltransferase reaction the assay converts UDP to ATP, which can then be used in a coupled reaction with Ultra-Glo™ Luciferase to produce light (see figure). This assay will help researchers learn more about protein glycosylation and help speed up the development of future therapeutics.
References
- IUPAC Gold Book- Glycans (http://goldbook.iupac.org/G02645.html)
- National Research Council of the National Academies 2012 Report, “Transforming glycoscience: A roadmap to the future”.
Latest posts by Promega (see all)
- Beyond Ozempic: The New Frontier of Obesity Research - April 18, 2025
- One Health and H5N1: Promega’s Commitment to Holistic Solutions - April 8, 2025
- Overcoming qPCR Inhibitors: Strategies for Reliable Quantification - March 13, 2025
