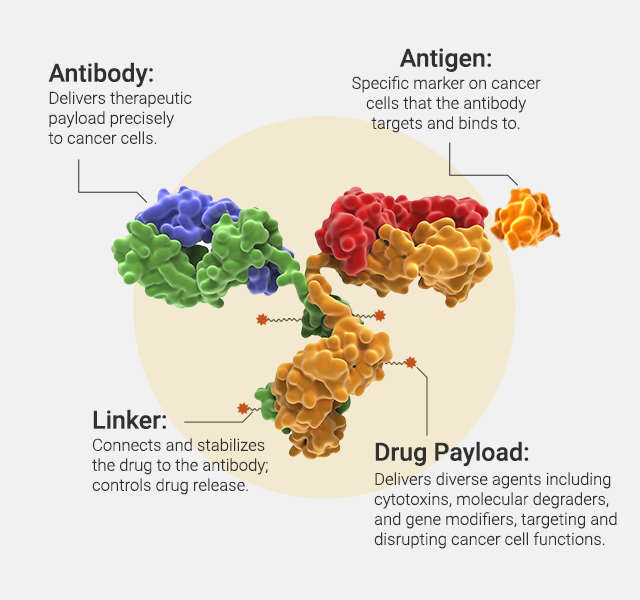
Antibody-drug conjugates (ADCs) are an increasingly powerful class of cancer therapeutics that combine the targeted precision of monoclonal antibodies with the cytotoxic potency of small-molecule drugs. By directing chemotherapy agents specifically to tumor cells, ADCs aim to maximize antitumor activity while minimizing damage to healthy tissues. One key challenge in ADC design is selecting the right target and payload—features that define efficacy, safety and resistance.
A new study published in Scientific Reports introduces OBI-992, an investigational ADC designed to target TROP2 (trophoblast cell surface antigen 2)—a transmembrane protein expressed in many epithelial cancers and associated with poor clinical outcomes. OBI-992 combines a novel anti-TROP2 antibody (R4702) with exatecan, a potent topoisomerase I (TOP1) inhibitor, tethered together by a cleavable hydrophilic linker. This study provides a comprehensive in vitro characterization of OBI-992, including its target binding profile, internalization behavior, resistance mechanisms and potential synergy with PARP inhibitors. Read further to see if it’s a promising, next-generation ADC candidate. PARP inhibitors. Read further to see if it’s a promising, next-generation ADC candidate.
What Sets OBI-992 Apart?
Competitive binding assays showed that OBI-992 binds a unique epitope on the cysteine-rich domain (CRD) of TROP2. This distinguishes it from benchmark TROP2-targeting ADCs like sacituzumab govitecan (SG) and datopotamab deruxtecan (Dato-DXd), which bind the cysteine-poor domain (CPD). Targeting the CRD could offer therapeutic advantages, particularly in tumors with mutations in the CPD that may lead to resistance.
OBI-992 pairs this novel antibody (R4702) with exatecan—a highly potent topoisomerase I (TOP1) inhibitor—linked via a cleavable hydrophilic linker, supporting efficient delivery and payload release inside tumor cells.
Key Findings from the Study
1. Target Binding and Internalization
For ADCs to induce cytotoxicity, they must be internalized by target cells to release their therapeutic payloads. Internalization assays demonstrated that OBI-992 and its parent antibody R4702 were efficiently internalized by cancer cells, including BxPC-3 and NCI-N87 lines. Internalization behavior was consistent between the antibody and ADC versions, indicating that the conjugation process did not impair cellular uptake.
2. Cytotoxicity and DNA Damage
To evaluate the relative potency of TOP1-targeting ADCs, the cytotoxicity of exatecan, DXd, and SN-38 was measured across different cancer cell types.
OBI-992 uses exatecan, a potent TOP1 inhibitor, as its payload. In cytotoxicity assays, exatecan demonstrated 2–5x greater potency than DXd or SN-38 across multiple cancer cell types. The study used the Promega CellTiter-Glo® Luminescent Cell Viability Assay and CellTiter-Glo® 3D Cell Viability Assay to evaluate ADC potency in 2D and 3D cultures, respectively. These assays provided ATP-based quantification of viable cells, enabling precise comparisons of drug efficacy.
In 3D prostate cancer spheroid models, OBI-992and Dato-Dxd both achieved strong cytotoxic effects, whereas SG had higher cellular toxicity at lower levels. CellTiter-Glo® 3D was particularly valuable in measuring ATP content and metabolic health in dense spheroid structures, where traditional viability assays may struggle.
3. Resistance Mechanisms

To evaluate potential resistance, the researchers developed DLD-1 colorectal cancer lines resistant to SG, Dato-DXd, or OBI-992. A western blot technique was used and notably, BCRP expression—an efflux pump associated with multidrug resistance—was upregulated in SG- and Dato-DXd-resistant cells, but not in OBI-992-resistant cells. This finding aligns with the known properties of exatecan, which is a weak BCRP substrate, suggesting OBI-992 may be less susceptible to efflux-based resistance mechanisms that can occur with chemotherapy drugs.
In contrast to Dato-DXd, which showed substantial TROP2 downregulation in resistant lines, OBI-992 maintained higher TROP2 expression thereby potentially preserving its therapeutic targeting ability even after prolonged exposure. Additionally, no differences in P-gp, another ABC transporter, were detected amongst the three resistant cell lines.
4. Synergy with PARP Inhibitors
The study also investigated OBI-992 in combination with PARP inhibitors, a class of drugs that impair DNA repair pathways, which have been shown to exhibit synergistic effects with poly (ADP-ribose) polymerase (PARP) inhibitors. Researchers used a screening study to assess cytotoxicity of exatecan with four FDA-approved PARP inhibitors. Co-treatment with talazoparib significantly enhanced cytotoxicity in breast, gastric and pancreatic cancer cell lines. Western blotting confirmed increased DNA damage (via γH2AX expression) in cells receiving combination treatment, suggesting a synergistic effect. These results point to a broader therapeutic window for OBI-992 beyond BRCA-mutant tumors.
Summarization: Implications for ADC Development
OBI-992 displays several characteristics that make it a strong contender in the ADC landscape:
- Distinct Epitope Targeting: Binding a unique CRD region may reduce cross-resistance with existing ADCs.
- Reduced Resistance Risk: Lack of BCRP upregulation in resistant cells supports potential use in multidrug-resistant cancers.
- Potent Payload: Exatecan demonstrates strong cytotoxic activity with a lower required DAR (drug-to-antibody ratio).
- Effective in 3D Models: The CellTiter-Glo® assays provided reliable insights into ADC activity in spheroids—critical for mimicking in vivo environments.
Conclusion
This study highlights how a variety of tools—including internalization tracking, cytotoxicity profiling and resistance studies—can shape ADC development. It also illustrates the utility of Promega assays in evaluating both ADC potency and mechanism of action. Tools like the CellTiter-Glo® and RealTime-Glo™ Assays, as well as internalization assays are central to our ADC Discovery and Development portfolio, designed to streamline research from target validation through to functional screening.
References:
- Chang, T.-Y., Lin, C.-J., Wen, S.-N., Wu, Y.-C., Wei, C.-Y., Huang, J.-Y., Tsao, Y.-H., Chen, Y.-J., Tang, W.-C., Wu, Y.-C., Lee, W.-H., Huang, T.-Y., Kuo, T.-M., Li, W.-F., & Lai, M.-T. (2025). Preclinical evaluation of a novel antibody–drug conjugate OBI-992 for Cancer therapy. Scientific Reports, 15(1). https://doi.org/10.1038/s41598-025-92697-z
Latest posts by Shannon Sindermann (see all)
- What Makes OBI-992 Different? A Closer Look at This TROP2 Antibody Drug Conjugate - April 29, 2025
- What 32,000 3D Spheroids Revealed About Culture Conditions - April 15, 2025
- Can Fungi Help Clean Up Environmental Contaminants? - March 20, 2025
