Two Is Better Than One
Redundancy equips us to survive. We have more than one lung or one kidney for a reason—if one organ in a pair gets damaged, we can still manage if the other is functional. At the cellular level, we have two copies of each chromosome in every non-germline cell. Each copy was inherited originally from a single sperm and ovum, which are “haploid” cells. Consequently, there are two copies of any given gene in non-germline “diploid” cells. In many cases, should one copy of a gene be damaged, the cell can still survive with the other, functional copy of a gene. In plants, this redundancy is common, and many plants exhibit polyploidy. In an extreme example of polyploidy, the large (by bacterial standards) but otherwise unassuming species Epulopiscium contains tens of thousands of copies of its genome.
However, not all pairs of genes have one copy serving as a backup. Sometimes, both copies of the gene are expressed, resulting in a high level of the gene product within a cell. If one copy of the gene is inactivated through loss-of-function (LoF) mutations, expression levels are reduced by half. This condition, termed haploinsufficiency, can have pathological consequences that manifest in diseases ranging from limb malformations to cancer.
Gene therapy approaches to correct haploinsufficiency have largely favored the use of recombinant adeno-associated virus (rAAV) to deliver a corrected copy of the gene into cells. This approach has the advantage of generating long-lasting expression with a low incidence of integration into the host genome. Unfortunately, the virus has a relatively small packaging capacity: it can accept genes up to 3.5 kb, after accounting for required regulatory sequences. It can also induce ectopic expression of the transferred gene, i.e., expression in non-targeted tissues or organs.
CRISPR to the Rescue
These days, it’s difficult to talk about gene therapy without mentioning CRISPR. (For an overview of how CRISPR gene-editing technology works, see A Crash Course in CRISPR and Five Ways to Explain CRISPR Without Delivering a Lecture elsewhere on this blog.) The solution to haploinsufficiency that first comes to mind is simple, conceptually: just repair the defective gene by editing the mutated region, and restore its function. Sounds easy, doesn’t it?
The problem with this approach is that, while feasible, it would require customizing an editing strategy for each mutation in each gene that contributes to a disease phenotype. Matharu et al. devised a better way—increase expression of the “good” copy of the gene to the level that would occur if both copies were active. Their unique twist on CRISPR technology is a CRISPR activation (CRISPRa) method that exploits the targeting mechanism of a guide RNA (gRNA) to direct the complex to a regulatory region of a gene, such as a promoter or enhancer. In this strategy, the heavy lifting is done by a novel nuclease-deficient Cas9 protein fused to VP64, a transcriptional activator derived from herpes simplex virus.
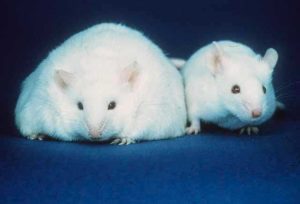
The researchers tested their CRISPRa strategy in mice by targeting obesity caused by haploinsufficiency of two genes. The Sim1 gene encodes a transcription factor active in the developing kidney, central nervous system and hypothalamus. LoF mutations in the human Sim1 gene lead to severe obesity. SIM1 also acts downstream of the human leptin-melanocortin signaling pathway that governs food intake and body weight. The melanocortin 4 receptor gene (Mc4r) is one target of SIM1 activation, and downregulation of SIM1 results in significantly reduced levels of Mc4r mRNA in the paraventricular nucleus (PVN) of the hypothalamus. In mice, haploinsufficiency for Mc4r results in obesity driven by hyperphagia (excessive hunger and food intake), hyperinsulinemia and hyperglycemia.
In Sim1+/− mice, CRISPRa targeting of either a Sim1 promoter or a distal enhancer rescued Sim1-mediated obesity. From RNA-Seq and chromatin immunoprecipitation sequencing (ChIP-Seq) results, the researchers found that this upregulation of Sim1 was highly tissue-specific and there were no observed off-target effects—a common occurrence in conventional CRISPR-based gene editing.
Further, Matharu et al. used rAAV-mediated delivery of CRISPRa to the hypothalamus in Sim1+/− and Mcr4+/− mice. They observed a substantial reduction in weight gain in both sets of mice.
The CRISPRa strategy described in this publication holds promise for treating diseases caused by haploinsufficiency. The opposite approach—CRISPR-mediated inactivation (CRISPRi) could also be employed in diseases caused by overexpression of a gene, such as in many types of cancer.
Reference:
Matharu, N. et al. (2019) CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363, 246.
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023
