 One piece of advice you will get from our Technical Services and R&D Scientists with regard to cell-based assays is to pay attention to what you are doing. Sounds obvious, but sloppiness can easily enter into the equation. Do you always count your viable cells with a hemocytometer and trypan blue exclusion before you split a culture? Do you always make sure that each well of your plate or plates contain the same number of cells? Two of our scientists, Terry Riss and Rich Moravec, published a paper demonstrating how decisions you make in experimental setup can ultimately affect the results you obtain. A natural consequence of this is difficulty replicating experiments if you didn’t pay attention to the details during the initial experimental setup.
One piece of advice you will get from our Technical Services and R&D Scientists with regard to cell-based assays is to pay attention to what you are doing. Sounds obvious, but sloppiness can easily enter into the equation. Do you always count your viable cells with a hemocytometer and trypan blue exclusion before you split a culture? Do you always make sure that each well of your plate or plates contain the same number of cells? Two of our scientists, Terry Riss and Rich Moravec, published a paper demonstrating how decisions you make in experimental setup can ultimately affect the results you obtain. A natural consequence of this is difficulty replicating experiments if you didn’t pay attention to the details during the initial experimental setup.
Cell Density Per Well Affects Response to Treatment
To demonstrate how cell density can affect your data, Riss and Moravec set up parallel plates with three different cell densities of HepG2 cells and measured the response to tamoxifen. The lower the cell density per well, the more pronounced the effect of the tamoxifin on the cells. Higher density cells were more resistant to tamoxifen.
Cell Density of Stock Culture Affects Response to Treatment
To demonstrate how the density of your stock culture can affect results, Riss and Moravec grew up a culture of the suspension cell line HL-60. The cells were split and cultured at different densities for 4 days. After harvesting, the cells were counted and then plated at 25,000 cells/well and treated with vinblastine. The cells cultured at the lowest density responded more rapidly to the treatment. They also exhibited a higher overall ATP content.
What’s the Explanation?
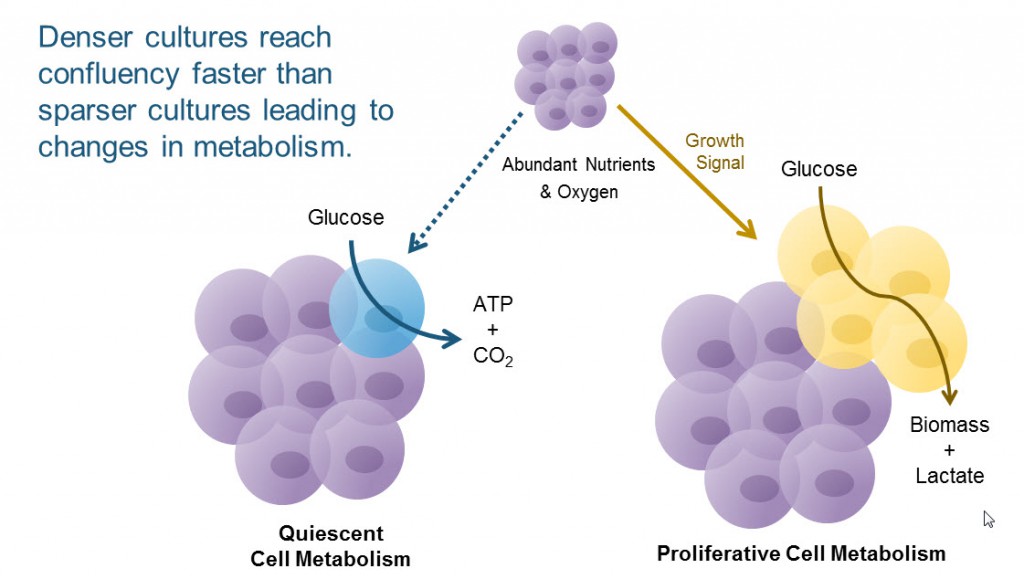
An explanation for how cell density per well and cell density of stock culture affect your data could simply be a shift in metabolic status. Higher-density cultures reach confluency faster than sparse cultures, and confluency can shift the cells from a proliferative metabolism to a quiescent metabolism. As our cell culture experts will tell you, reproducibility from experiment-to-experiment depends on how well you replicate what you’ve done in the past.
Obsessive note taking can be your friend. So, grow your cells identically for each experiment, and plate the same number of cells per well.
Here’s the Paper
Riss, T.L. and Moravec, R.A. (2004) Use of Multiple Assay Endpoints to Investigate the Effects of Incubation Time, Dose of Toxin, and Plating Density in Cell-Based Cytotoxicity Assays. Assay Drug Dev. Tech. 2, 51-62.

So plating at higher density you will have reduced response to drug treatment? If you plate some well at one cancer cell density and others at double the cancer cell density, would you expect the wells with double the cell density to exhibit a reduced response to anti-cancer drug treatment?
Hello Sukhi,
Yes, the data in the paper would imply that treating different densities of cells with the same treatment would result in density-dependent responses to the treatment. The key point here is that you should strive to be consistent from experiment to experiment with regard to how you handle the cells to increase reproducibility in your work.
Please let me know if you have more questions or need further clarification.
Regards,
Kyle