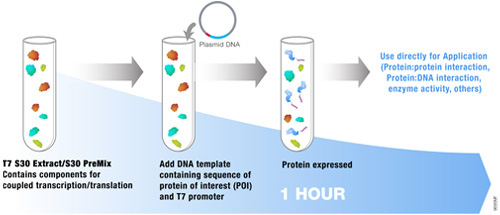
 Many applications require amounts of protein that cannot be obtained using a eukaryotic cell-free expression system. As an alternative, a prokaryotic system can be used when this need arises. The E. coli S30 T7 High-Yield Protein Expression System is designed to express up to 500μg/ml of protein in 1 hour from plasmid vectors containing a T7 promoter and a ribosome binding site. The protein expression system provides an extract that contains T7 RNA polymerase for transcription and is deficient in OmpT endoproteinase and lon protease activity. All other necessary components in the system are optimized for protein expression. This results in greater stability and enhanced expression of target proteins.The following references highlight the use of this system for a variety of unique applications:
Many applications require amounts of protein that cannot be obtained using a eukaryotic cell-free expression system. As an alternative, a prokaryotic system can be used when this need arises. The E. coli S30 T7 High-Yield Protein Expression System is designed to express up to 500μg/ml of protein in 1 hour from plasmid vectors containing a T7 promoter and a ribosome binding site. The protein expression system provides an extract that contains T7 RNA polymerase for transcription and is deficient in OmpT endoproteinase and lon protease activity. All other necessary components in the system are optimized for protein expression. This results in greater stability and enhanced expression of target proteins.The following references highlight the use of this system for a variety of unique applications:
Loh, E. et al. (2011) An unstructured 5′-coding region of the prfA mRNA is required for efficient translation. Nuc. Acids. Res. (online) Examines the effect of upstream codon sequence/length on the correct ribosome binding and translation initiation of the pfrA protein.
Mitsuhashi, H. et al. (2010) Specific phosphorylation of Ser458 of A-type lamins in LMNA-associated myopathy patients. J. Cell. Sci. 123, 3893–900 By creating a series of mutations in the protein lamin A, Akt1 phosphorylation sites were determined.
Halvorsen, E. et al. (2011) Txe, an endoribonuclease of the enterococcal Axe-Txe toxin-antitoxin system, cleaves mRNA and inhibits protein synthesis. Microbiology 157, 387–97. S30 High Yield System was used to characterize the inhibitory effect of Txe toxin on protein expression.
Mo, P. et al. (2010) MDM2 mediates ubiquitination and degradation of activating transcription factor 3. J. Biol. Chem. 285, 26908–15. By using in vitro pull down experiments the researchers characterized the binding of AFT3 to MDM2 leading to the proteolysis of AFT3 system by ubiquitination.
