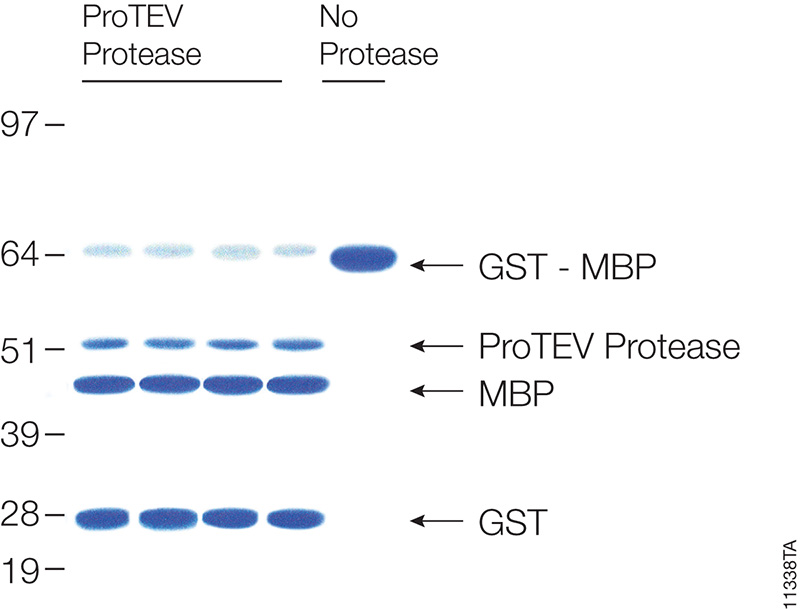
Many proteins are expressed as fusion partners with affinity tags, such as the HaloTag® fusion, glutathione-S-transferase (GST) or maltose binding protein (MBP), to selectively bind the proteins using affinity purification resins. While such resins yield high-purity protein quickly, the large affinity tags are undesirable for some downstream applications. Most expression vectors are designed with a specific protein cleavage site between the two fusion partners to remove the affinity tag after purification. ProTEV Protease recognizes a rare amino acid sequence, EXXYXQ, where X is any amino acid, and cleaves after the glutamine residue.
ProTEV Plus functions over a broad pH and temperature range. In a recent study the enzymatic activity of ProTEV Plus in the presence of various compounds (Table 1) commonly found in protein purification protocols were evaluated.
Continue reading “ProTEV Protease Compound Compatibility Analysis”