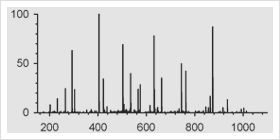
One of the approaches to identify proteins by mass spectrometry includes the separation of proteins by gel electrophoresis or liquid chromatography. Subsequently the proteins are cleaved with sequence-specific endoproteases. Following digestion the generated peptides are investigated by determination of molecular masses or specific sequence. For protein identification the experimentally obtained masses/sequences are compared with theoretical masses/sequences compiled in various databases.
Are you looking for proteases to use in your research?
Explore our portfolio of proteases today.
Nonspecific proteases such as pepsin, proteinase K, elastase and thermolysin can offer an alternative to traditional sequence-specific proteases for certain applications. The following references illustrate the use of nonspecific proteinases for the mass spec analysis of proteins:
Papasotiriou, D. et al. (2010) Peptide mass fingerprinting after less specific in-gel proteolysis using MALDI-LTQ-Orbitrap and 4-chloro-alpha-cyanocinnamic acid. J. Proteome. Res. 9, 2619–29. This reference demonstrates the use of either chymotrypsin, elastase, trypsin or proteinase K in combination with matrix CHCA for increase peptide identification and sequence coverage using MALDI.
Neue, K. et al. (2011) Elucidation of glycoprotein structures by unspecific proteolysis and direct nanoESI mass spectrometric analysis of ZIC-HILIC-enriched glycopeptides. J. Proteome. Res. 10, 2248–60. Notes use of thermolysin or elastase in combination with ZIC-HILIC enrichment as alternative method for the characterization of glycopeptides.
Baeumlisberger, D et al. (2011) Simple dual-spotting procedure enhances nLC-MALDI MS/MS analysis of digests with less specific enzymes. J. Proteome. Res. 10, 2889–94. Data noted that samples digested with elastase followed by nLC separation and subsequent alternative spotting on both MALDI-LTQ-Orbitrap and MALDL-TOF/TOF instruments resulted in 32% additional peptides.