
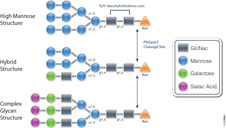
Food allergies are increasing worldwide and becoming a public health issue, especially among children are concerned. Children have a higher prevalence of food allergies, with about 4–8%, compared to adults (1–5%). Currently antibody-based methods such ELISA (enzyme-linked immunosorbent assay) are the primary method for food allergen analysis. In most cases antibodies are only available for single well-known allergens. Often those that are commercially available are poorly characterized resulting cross-reactivity that leads to false-positive results in diagnostic tests.
A recent publication (1) presented a review of an alternative technology based on mass spec (i.e., multiple reaction monitoring, MRM) that circumvents the drawbacks of antibody based methods. MRM allows precise quantitative determination of target proteins in complex samples with broad dynamic range. MRM also provides quantification of different isoforms. It is noted that tryptic digestion followed by mass spec analysis, has already identified several unique peptides for different allergens, including those found in crustaceans, eggs, fish, peanuts, soy and wheat. In summary the challenge is now to select the appropriate tryptic signature peptide(s) for the respective allergen and to develop well characterized standards (i.e., isotope labeled standards) to ensure accurate quanititation.
Citation:
Koeberl, M et al. (2014) Next Generation of Food Allergen Quantification using Mass Spectrometric Systems J. Proteome Research 13, 3499–509.
 I am fascinated by all the ways that scientists are taking sensitive techniques and using them to look into our past. For example,
I am fascinated by all the ways that scientists are taking sensitive techniques and using them to look into our past. For example, 