Most of us have experienced an inflammatory response at some point in our lives. Fever, achy joints, swelling around a scrape or cut, all of these are forms of inflammatory response. Inflammation is the body’s response to infection or tissue damage and acts to limit harm to the rest of the body. A key player in the inflammation process is a group of protein complexes call inflammasomes. The term “inflammasome” was first used in 2002 by researchers in Switzerland (1) to refer to a caspase-activating protein complex. We now know that inflammasomes are cytosolic multiprotein platforms that assemble in response to pathogens and other signals. Inflammasome assembly results in the processing of the inactive procaspase-1 into the active cysteine-protease enzyme, caspase-1. Caspase-1, in turn, activates the proinflammatory cytokines Interleukins IL-1β and IL-18. In addition, caspase-1 is also required for pyroptosis, which is an inflammatory form of cell death that combines the characteristics of apoptosis (fragmented DNA) and necrosis (inflammation and cytokine release) and is frequently associated with microbial infections.
Inflammasome complexes are made up of scaffolding sensor proteins (NLR, AIM2, ALR), and an adaptor protein containing a caspase activation and retention domain (CARD) and inactive procaspase-1. Most inflammasomes are formed with one or two NLRs (NOD-like receptors). However, non-NLR proteins such as AIM2 (absent in melanoma 2) and pyrin can also form inflammasomes. The different sensor proteins are activated by different types of outside stimuli, and inflammasomes are loosely sorted into families based on the protein forming these sensors.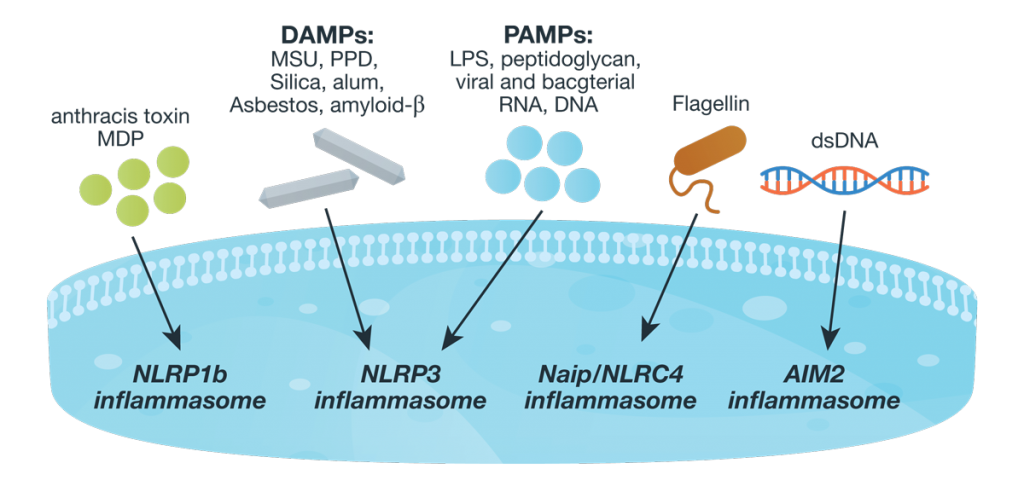 Continue reading “Inflammasomes: Peeking Inside the Inflammatory Process”
Continue reading “Inflammasomes: Peeking Inside the Inflammatory Process”
