 Many different polypeptide fusion partners or affinity tags have been developed to facilitate purification of target proteins. The most commonly used tag for the purification and detection of recombinant expressed proteins is the His tag. Cloning vectors designed to generate His-tagged proteins contain 5–10 histidine residues at either the C- or N terminus of the expressed protein. The His tag adds only 0.84kDa to the mass of the protein and is nonimmunogenic. Also, because the tertiary structure of the tag is not important for purification, His-tagged proteins can be purified using native or denaturing conditions. The affinity of histidine residues for immobilized nickel allows selective purification of His-tagged proteins. The MagneHis™ Ni-Particles can bind up to 1mg of His-tagged protein per milliliter of particles providing a fast, efficient method for purifying His-tagged proteins with high yield and low background in a highly scalable format.
Many different polypeptide fusion partners or affinity tags have been developed to facilitate purification of target proteins. The most commonly used tag for the purification and detection of recombinant expressed proteins is the His tag. Cloning vectors designed to generate His-tagged proteins contain 5–10 histidine residues at either the C- or N terminus of the expressed protein. The His tag adds only 0.84kDa to the mass of the protein and is nonimmunogenic. Also, because the tertiary structure of the tag is not important for purification, His-tagged proteins can be purified using native or denaturing conditions. The affinity of histidine residues for immobilized nickel allows selective purification of His-tagged proteins. The MagneHis™ Ni-Particles can bind up to 1mg of His-tagged protein per milliliter of particles providing a fast, efficient method for purifying His-tagged proteins with high yield and low background in a highly scalable format.
Bacterial expression of recombinant His-tagged proteins is a common technique. However, use of other systems, such as Sf9 insect cells,or HeLa or CHO mammalian cells for expression of recombinant proteins either intracellularly or secreted into the culture medium is increasing. These eukaryotic expression systems may allow more natural processing and modification of recombinant His-tagged proteins.
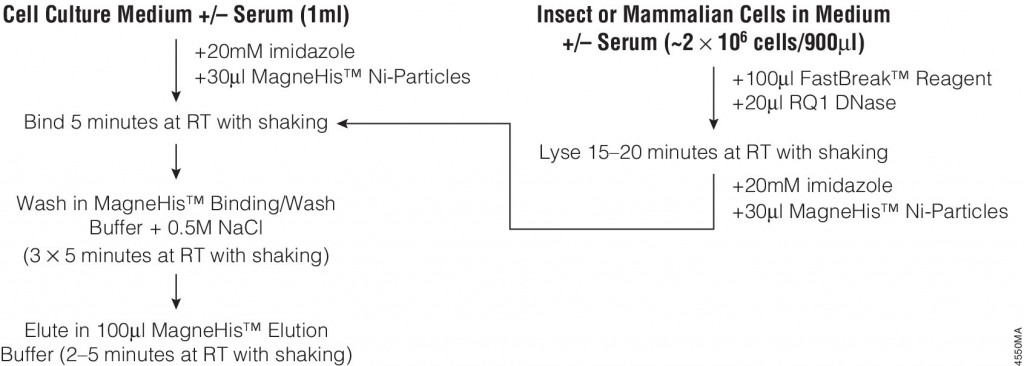
The following article: illustrates the use of FastBreak™ Cell Lysis Reagent and the MagneHis™ Protein Purification System with insect and mammalian cell lysates. Proteins are purified from culture medium in the presence or absence of serum with only minior modifications to the standard protocol for bacterial cultures are required for purification from these diverse sources.