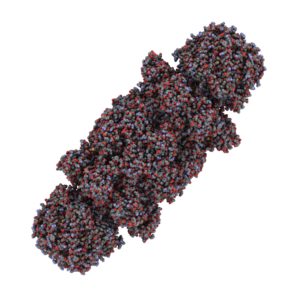
CD73 also known as 5´-Ectonucleotidase (NT5E) is a membrane-anchored protein that acts at the outer surface of the cell to convert AMP to adenosine and free phosphate. CD73 activity is associated with immunosuppression and prometastatic effects, including angiogenesis. CD73 is highly expressed on the surfaces of many types of cancer cells and other immunosuppressive cells (1). A recent study by Quezada and colleagues showed that the high concentration of adenosine produced by the CD73-catalyzed reaction on glioblastoma multiforme cells, which are characterized by extreme chemoresistance, triggered adenosine signaling and in turn, the multi-drug resistance (MDR) phenotype of these cells (2).
Because of the roles of adenosine in immunosuppression, angiogenesis and MDR phenotypes, CD73 (NT5E) is an attractive therapeutic target. However, the current methods of assaying for the ectonucleotidase activity, HPLC and a malachite green assay, are cumbersome and not suited to high-throughput screening. The HPLC assay is expensive and difficult to automate and miniaturize (3). The malachite green assay is sensitive to phosphate found in media, buffers and other solutions used in the compound-screening environment.
To address the problem of developing a reliable high-throughput screening assay for CD73, Sachsenmeier and colleagues (3) looked to a luminescent ATP-detection reagent.
Continue reading “Screening for Inhibitors of CD73 (5´-ectonucleotidase) Using a Metabolite Assay” Like this:
Like Loading...

 Life is complicated. So is death. And when the cells in your multiwell plate die after compound treatment, it’s not enough to know that they died. You need to know how they died: apoptosis or necrosis? Or, have you really just reduced viability, rather than induced death? Is the cytotoxicity you see dose-dependent? If you look earlier during drug treatment of your cells, do you see markers of apoptosis? If you wait longer, do you observe necrosis? If you reduce the dosage of your test compound, is it still cytotoxic?
Life is complicated. So is death. And when the cells in your multiwell plate die after compound treatment, it’s not enough to know that they died. You need to know how they died: apoptosis or necrosis? Or, have you really just reduced viability, rather than induced death? Is the cytotoxicity you see dose-dependent? If you look earlier during drug treatment of your cells, do you see markers of apoptosis? If you wait longer, do you observe necrosis? If you reduce the dosage of your test compound, is it still cytotoxic? 