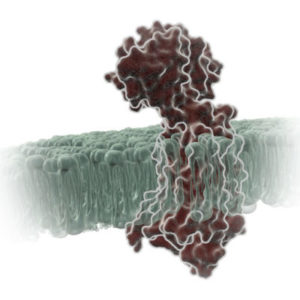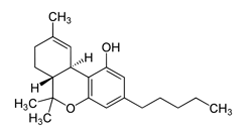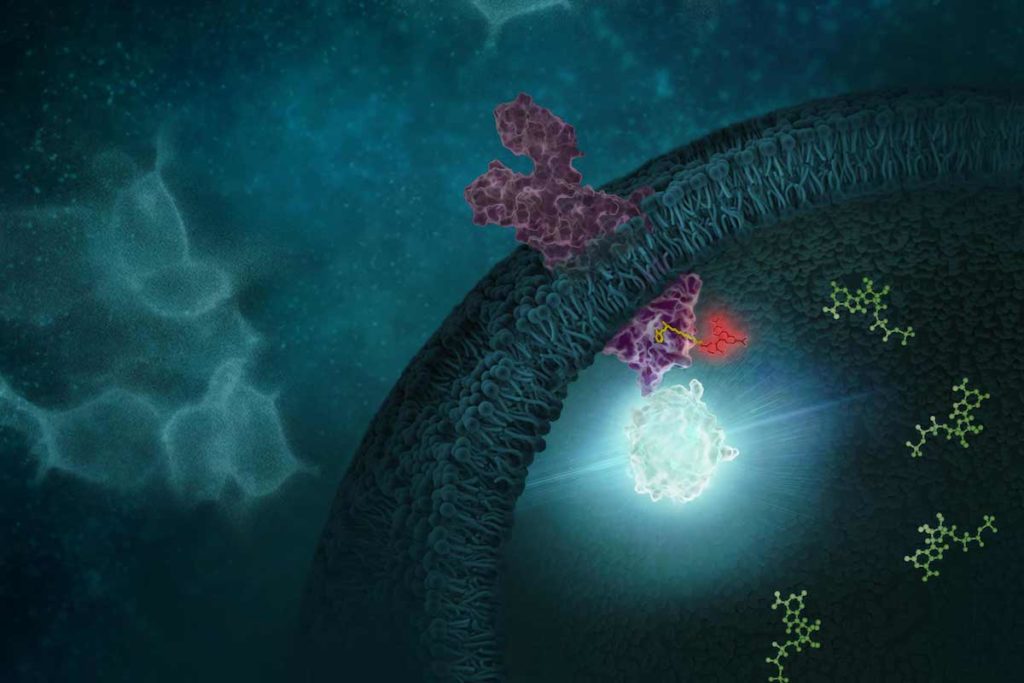
G protein-coupled receptors (GPCRs) comprise a large group of cell surface receptors, characterized by the unique structural property of crossing the cell membrane seven times. They respond to a diverse group of signaling molecules, such as peptides, neurotransmitters, cytokines, hormones and other small molecules (1). Upon activation, GPCRs interact with GTP-binding (G) proteins and arrestins to regulate a wide variety of signaling pathways. This broad range of functions makes GPCRs attractive targets for drug discovery. The importance of GPCR research was highlighted in 2012, with the Nobel Prize in chemistry being awarded to Robert Lefkowitz and Brian Kobilka “for studies of G-protein–coupled receptors”.
Based on structure and function, GPCRs are categorized into six classes, A–F. The class A GPCRs, or rhodopsin-like receptors, have been studied extensively due to their association with many types of diseases (2). Within the class A GPCRs is a group that share a highly conserved structural motif (3) and respond to chemokines—small “chemotactic cytokines” that stimulate cell migration, especially that of white blood cells (4). A subfamily of class A GPCRs respond to chemokines that have two cysteine residues near the N-terminus, known as CC chemokines. GPCRs activated by CC chemokines are called CC chemokine receptors or CCRs, and these interactions have been implicated in both pro- and anti-cancer pathways (5).
Continue reading “GPCRs and PROTACs: New Approaches for Designing More Effective Drug Candidates” GPCRs
GPCRs