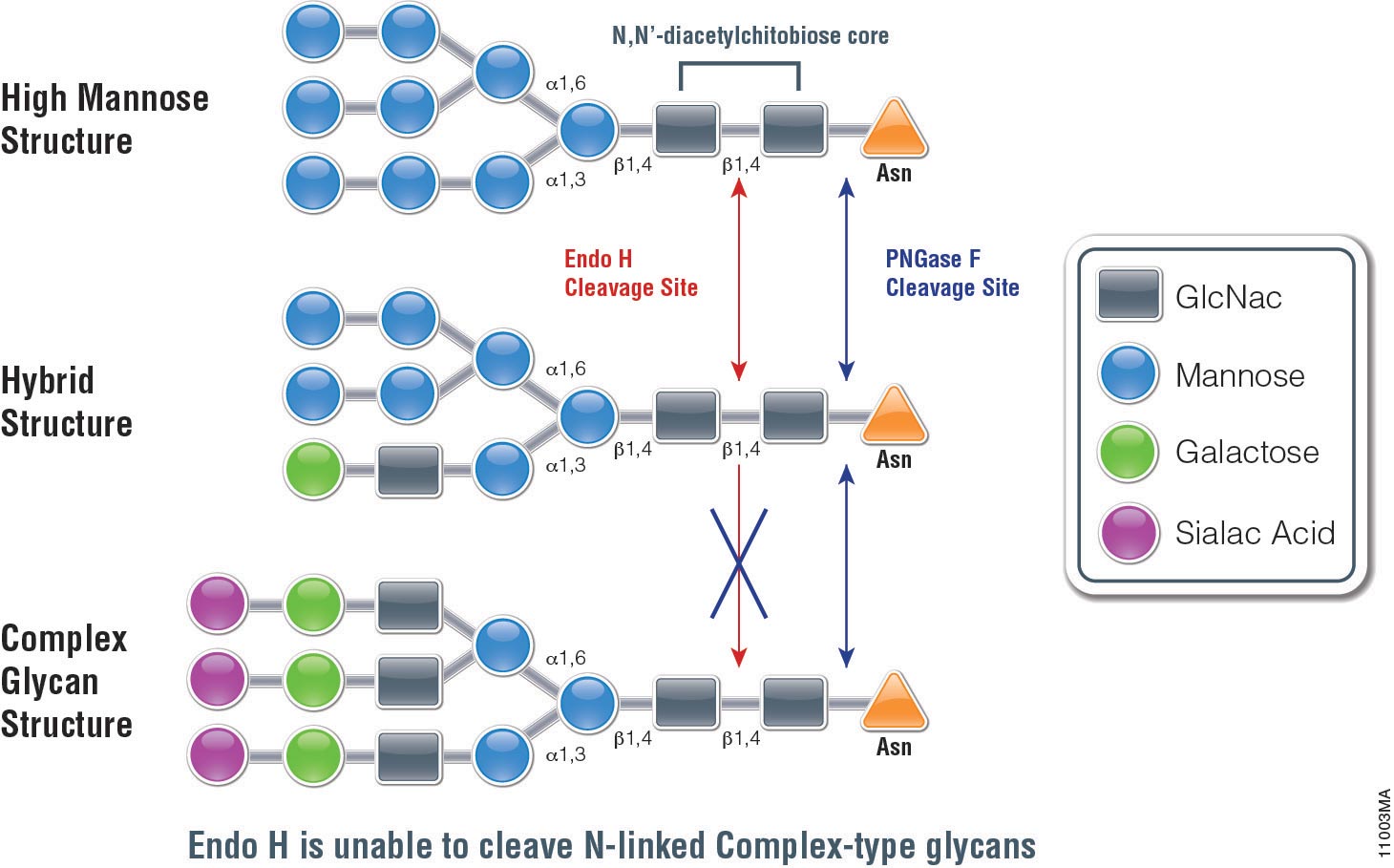
 Endo H (Endo-ß-N-acetylglucosaminidase H) is a 29,000 dalton protein with optimal activity between pH 5 and 6. In contrast to PNGase F, which cleaves all N-linked glycans at the site of attachment to Asparagine (Asn), (with the exception of those with fucose attached to the core GlcNac moieties), Endo H hydrolyses the bond connecting the two GlcNac groups that comprise the chitobiose core (see Figure 1.). In addition, Endo H cleaves high mannose and hybrid glycans, whereas complex glycans (those with more than 4 different sugar types per glycan chain, including the GlcNac groups) are resistant to hydrolysis.
Endo H (Endo-ß-N-acetylglucosaminidase H) is a 29,000 dalton protein with optimal activity between pH 5 and 6. In contrast to PNGase F, which cleaves all N-linked glycans at the site of attachment to Asparagine (Asn), (with the exception of those with fucose attached to the core GlcNac moieties), Endo H hydrolyses the bond connecting the two GlcNac groups that comprise the chitobiose core (see Figure 1.). In addition, Endo H cleaves high mannose and hybrid glycans, whereas complex glycans (those with more than 4 different sugar types per glycan chain, including the GlcNac groups) are resistant to hydrolysis.
The unique specificty of Endo H and PNGase F can be used to monitor protein trafficking. Basic N-Glycosylation occurs in the endoplasmic reticulum. Proteins in this stage are sensitive to Endo H digestion. If proteins have entered the Golgi body where additional modifications occur to the glycan, they are resistant to Endo H digestion.
The following references illustrate this application:
- Taner, S. et al. (2011) Interactions of NK cell receptor KIR3DL1*004 with chaperones and conformation-specific antibody reveal a functional folded state as well as predominant intracellular retention. J. Immunol. 186, 62–72
- Beyer, S. et al. (2011) KT5823 differentially modulates sodium iodide symporter expression, activity, and glycosylation between thyroid and breast cancer cells. Endo. 152, 782–92.
- Chen, Z. et al. (2011) CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J. Exper. Med. 208, 2633–40.
- Iavarone, C. et al. (2011) A point mutation in the amino terminus of TLR7 abolishes signaling without affecting ligand binding. J. Immunol. 186, 4213–22.
- Guo, Y. et al. (2011) Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J. Exper. Med. 208, 2083–98.
