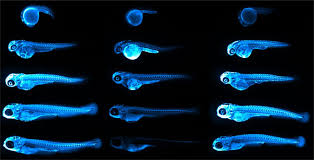
Credit: Figure 5.D of The LipoGlo reporter system for sensitive and specific monitoring of atherogenic lipoproteins by James Thierer, Stephen C. Ekker and Steven A. Farber.
Article licensed under Creative Commons Attribution 4.0 International License.
Cardiovascular diseases, or CVDs, are collectively the most notorious gang of cold-blooded killers threatening human lives today. These unforgiving villains, including the likes of coronary heart disease, cerebrovascular disease and pulmonary embolisms, are jointly responsible for more deaths per year than any other source, securing their seat as the number one cause of human mortality on a global scale.
One of the trademarks of most CVDs is the thickening and stiffening of the arteries, a condition known as atherosclerosis. Atherosclerosis is characterized by the accumulation of cholesterol, fats and other substances, which together form plaques in and on the artery walls. These plaques clog or narrow your arteries until they completely block the flow of blood, and can no longer supply sufficient blood to your tissues and organs. Or the plaques can burst, setting off a disastrous chain reaction that begins with a blood clot, and often ends with a heart attack or stroke.
Given the global prevalence and magnitude of this problem, there is a significant and urgent demand for better ways to treat CVDs. In a recent study published in Nature Communications, researchers at the Carnegie Institution for Science, Johns Hopkins University and Mayo Clinic are taking the fight to CVDs through the study of low-density lipoproteins (LDLs), the particles responsible for shuttling bad cholesterol throughout the bloodstream.
Apolipoprotein-B (ApoB) is a protein involved in lipid metabolism and serves as a primary structural constituent of atherogenic lipoproteins, including LDLs. Of the ApoB-Lipoproteins (ApoB-LPs), LDLs are the smallest and considered the chief drivers of atherosclerosis, as there are “several lines of evidence showing that people with a high number of small LDL particles are very likely to suffer from cardiovascular disease, even if those particles aren’t carrying very much bad cholesterol”, noted lead author of the study, James Thierer.
Although there are existing methods being used to isolate ApoB-LPs through assays measuring cholesterol and triglyceride content, these measurements provide very limited information on the ApoB-LP properties that actually affect atherogenic potential, including particle size distribution, localization and concentration.
In addition, these methods are ill-suited for characterizing ApoB-LPs, because they are unable to detect LPs that are located outside of the bloodstream. Though LPs have been studied a great deal in mammalian models, said models are not conducive to high-throughput drug screening, and the simpler models that are conducive to high-throughput screening (such as invertebrate models or cultured cells) are unable to replicate the complex, multi-organ physiology that composes the symphony that is ApoB-LP homeostasis. This, in turn, has made the task of targeting ApoB-LPs with pharmaceuticals exceptionally difficult.
To overcome the many pitfalls of the traditional LP particle characterization methods, the research team developed a whole new approach that directly measures and monitors several key determinants of LP particles. Enter: The LipoGlo System. LipoGlo is a sensitive and specific reporter system in which the team fused the engineered Promega reporter NanoLuc® luciferase to the endogenous apoB gene in zebrafish (the team’s model system of choice), effectively tagging each atherogenic LP with a luciferase molecule. The research team used the Promega Nano-Glo® Luciferase Assay System to quantify these tagged LPs.
Through the processing of its substrate molecule, furimazine, NanoLuc® luciferase generates a quantitative bioluminescent signal. In this study, this light signal allowed the team to monitor and characterize distinct features of ApoB-LPs in a variety of assays they developed, including LipoGlo electrophoresis (a gel-based assay to measure LP size), LipoGlo counting (a plate-based assay to quantify LPs) and LipoGlo microscopy (luminescent imaging that visualizes the localization of LPs).
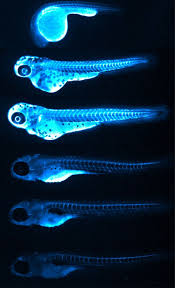
Credit: Figure 6.B of The LipoGlo reporter system for sensitive and specific monitoring of atherogenic lipoproteins by James Thierer, Stephen C. Ekker and Steven A. Farber.
Article licensed under Creative Commons Attribution 4.0 International License.
The team chose to use the zebrafish model due to some unique advantages it possesses over traditional models. Perhaps most important, “it is the only animal model conducive to high-throughput screening”, says Thierer, “A single lab can easily breed thousands or tens of thousands of zebrafish larvae every week, creating the opportunity to do thousands of experiments within a single week (which could take months or years in rodent models).”
Another major advantage of using this model is that larval zebrafish are transparent. “This has allowed us to image the localization of atherogenic lipoproteins in intact animals, revealing unexpected associations between LDL and the brain/spinal cord, as well as with tendinous tissues”, Thierer added, emphasizing that these connections could not have been made using traditional methods, which only extract LDL from blood.
In addition to these surprising new associations, the imaging also allowed them to confirm the patterns they predicted, which corresponded to LP-producing tissues, like the intestines, liver and yolk-syncytial layer. The study also revealed the gene pla2g12b (Phospholipase A2 group XII B) as a regulator of both size and number of ApoB-containing LPs that can be leveraged in the process of drug discovery.
These observations collectively highlight the importance of ApoB-LPs and the need for further evaluation of the physiological roles they may play elsewhere in the human body, not just the circulatory system. Fortunately, the LipoGlo system seems more than up to the challenge of taking on this expanding scope.
Why NanoLuc? “We selected NanoLuc for this application primarily due to its sensitivity”, said Thierer and co-author Steve Farber, “As there is only one copy of ApoB on every LDL particle, we decided to use genome engineering to insert a very sensitive light-emitting protein into ApoB”. There were concerns that a fluorescent reporter would not be bright enough to be detected, as live zebrafish have considerable background levels of autofluorescence.
The amount of starting material supplied by zebrafish also played a role in selecting NanoLuc® luciferase as their reporter. “Most assays for LDL from humans or mammals are performed from plasma, meaning that they begin with a large amount of relatively pure starting material,” Thierer and Farber explain. “This is nearly impossible in a larval zebrafish however, as their microscopic bloodstreams carry only a few nanoliters of plasma. NanoLuc fused to ApoB reproduced many of the same biochemical analyses performed on mammalian LDL using about 1,000 times less starting material.”
The team used NanoLuc® luciferase for their work, due to its stability, small size, brightness (~100 times brighter than firefly luciferase) and ATP-independence. Thierer and Farber elaborate that the “vast majority of LDL particles are extracellular, where ATP levels are quite low, so NanoLuc was our best option for a highly sensitive and ATP-independent reporter”.
Using the LipoGlo system, the researchers were able to demonstrate in their study that the LP profiles in larval zebrafish exhibited human-like responses to changes in dietary manipulations (high-fat feeding and fasting), genetic mutations (mtp-/- and apoc2-/-) and pharmaceuticals (Lomitapide), indicating the presence of highly conserved LP processing pathways between humans and zebrafish, and painting the larval zebrafish model system as an attractive one for further study of LP homeostasis.
Now that they’ve established a system to effectively measure the abundance and LDL particle size in zebrafish, what’s the next step for the team in the battle to bring down CVDs?
When asked about the future direction of their research, Thierer said “we are using this technology to screen for new genes and drugs that regulate LDL. Additionally, since we have discovered interesting localization patterns of LDL in larval zebrafish, we are using similar genome engineering approaches to generate fluorescent reporters of ApoB”. This will enable them to perform confocal super-resolution microscopy to help them determine the precise LDL localization patterns in tissues and cells.
With the help of this important research, the tables are finally starting to turn on CVDs, and the LipoGlo system will soon be the one striking fear into the hearts of that bloodthirsty gang of Cardiovascular Diseases.
Special thanks goes out to Jay Thierer, Steve Farber and Emily Williams at the Carnegie Institution for Science for their contributions to this blog post. Thank you for the important work you do and for giving us the opportunity to highlight it!
