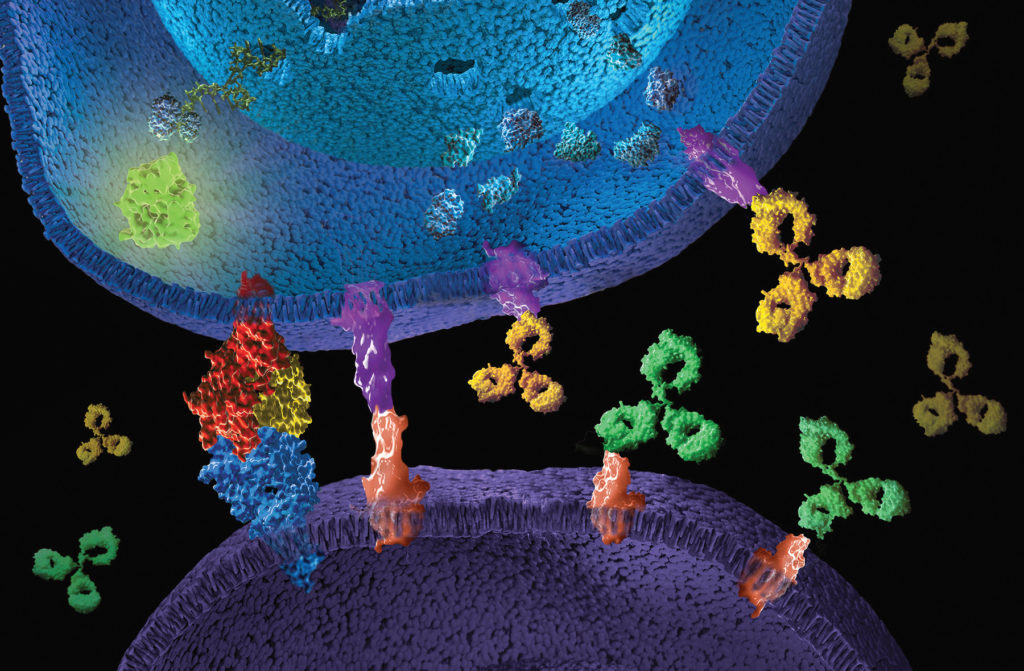
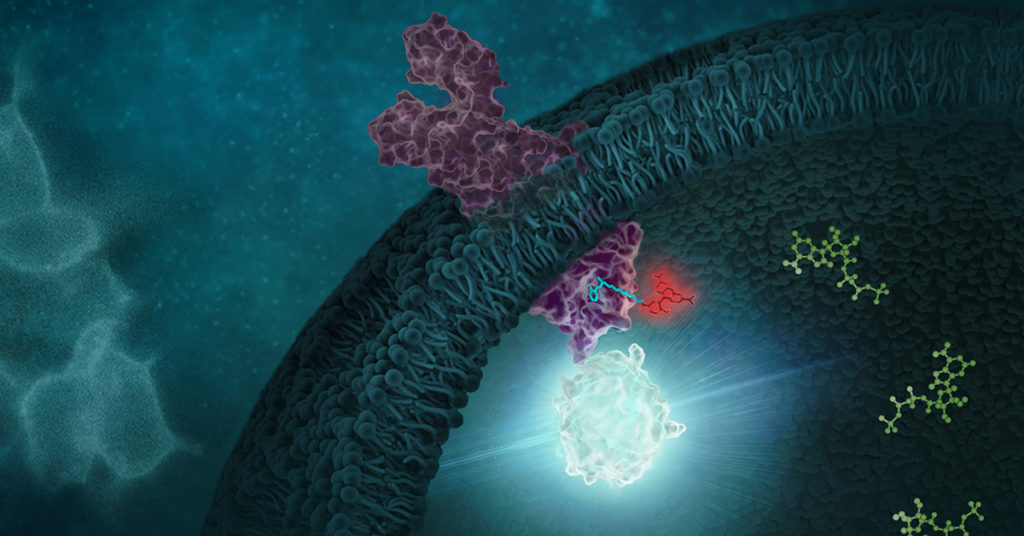
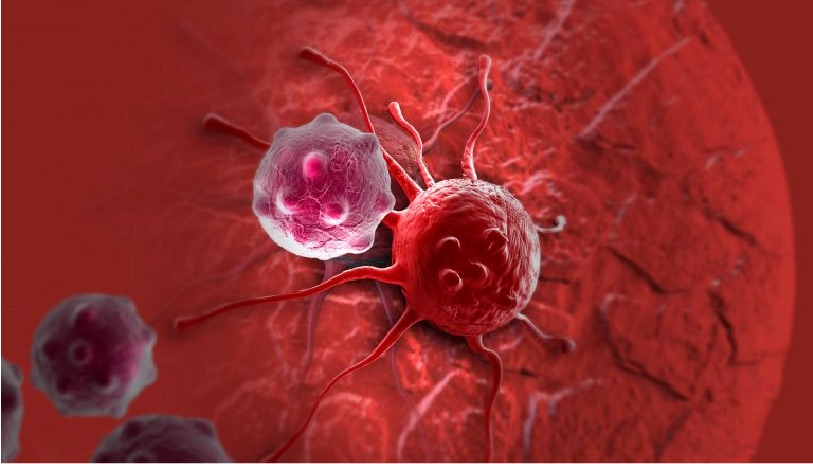
When we think of viruses, we often think of diseases, pandemics and death. Our impression of viruses is that they are “bad”. But viruses could also be a possible cure for the deadliest disease in modern history: cancer. The therapeutic effects of “good” cancer-killing oncolytic viruses have been documented over a century ago. Records from as early as 1904 described a 42-year old woman with acute leukemia who experienced temporary remission after an influenza infection. Other early reports showed spontaneous remission of Hodgkin lymphoma and Burkitt’s lymphoma after natural infections with the measles virus.
Despite the long history, oncolytic viruses have only recently gained momentum in the scientific community. Dr. Aldo Pourchet, CSO and co-founder of Omios Biologics—a biotech startup in the San Francisco Bay area—is determined to harness the power of oncolytic viruses to develop a new generation of cancer immunotherapy.
How Oncolytic Viruses Work
“One thing that we know for sure is that you need the immune system to fight the cancer,” says Pourchet. “You need to recruit the immune system, and probably the best thing we know for recruiting the immune system is viruses. Our immune system evolved to detect them immediately. That’s why we are still on Earth. It’s because we have been able to fight deadly viruses.”
Continue reading “Oncolytic Viruses: Models and Assays for Developing Viruses That Can Kill Cancer”