In early 2023, a type 2 diabetes medication, semaglutide (brand names Ozempic, Rybelsus), drew huge amounts of attention on social media and in popular culture. The reason? People were getting off-label (that is, not for treating type 2 diabetes) prescriptions of Ozempic to take advantage of one of its common side effects—measurable weight loss.
How does semaglutide and other drugs of its type manage diabetes on a molecular level, and what drives the weight loss effects?

Glucagon-Like Protein-1 (GLP-1) Receptor Agonists
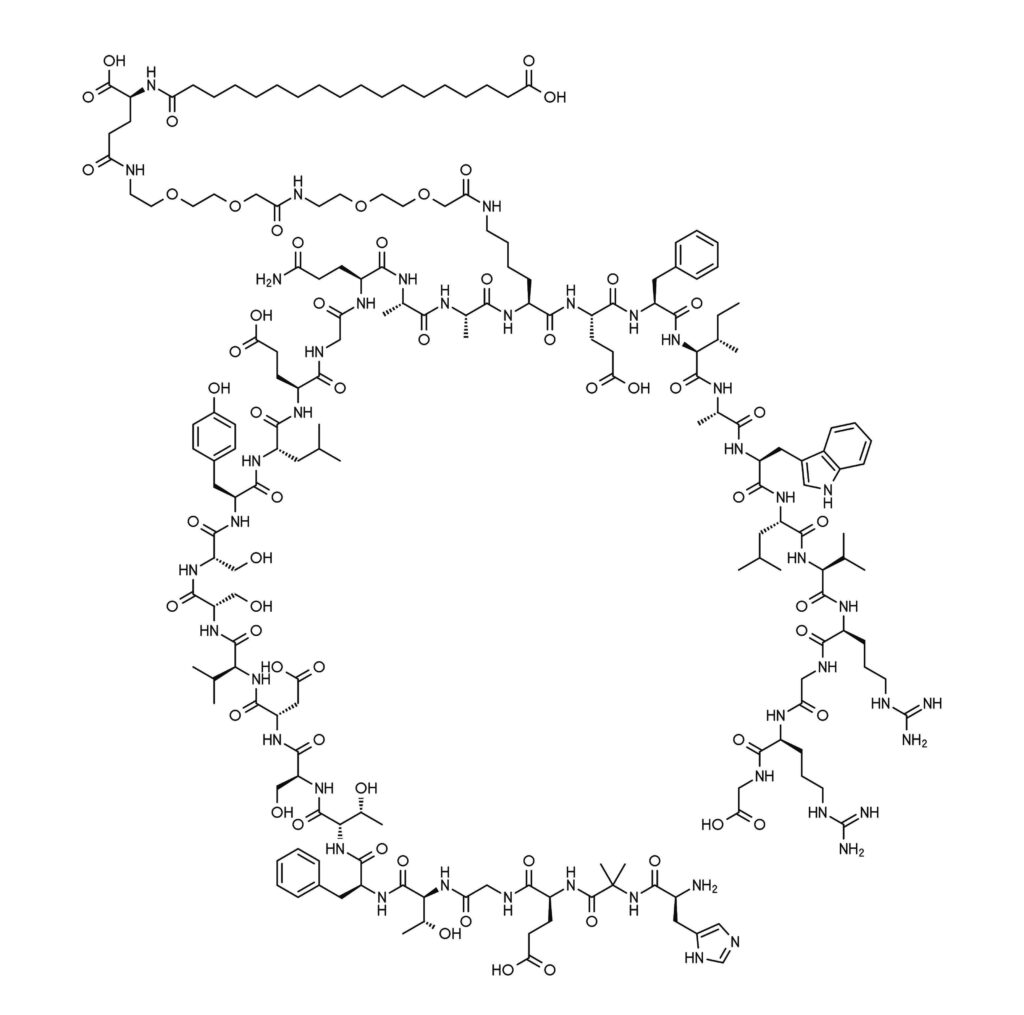
Semaglutide belongs to a class of compounds called glucagon-like protein-1 (GLP-1) receptor agonists, which have been approved in the United States since 2005 for managing blood sugar levels in type 2 diabetes (1). During some clinical investigations, it was observed that the GLP-1 receptor agonists caused weight loss in the study participants (2). A GLP-1 receptor agonist called liraglutide (brand name Saxenda) was first approved for chronic weight management in adults in 2014.
But then, in 2021, the FDA approved a formulation of semaglutide for weight loss (brand name Wegovy). Wegovy, like Ozempic, uses semaglutide as its active ingredient, but at a higher dose. In a clinical trial for Wegovy (clinical trial identifier: NCT03693430), patients who received a once weekly injection of Wegovy for two years experienced, on average, a 14.8% reduction in weight compared to 2.4% reduction with placebo. Study participants either were obese or were overweight with a weight-related condition (like hypertension) (3). In addition to its greater weight loss effects, semaglutide only requires a once weekly injection while liraglutide requires a daily injection (4).
As Wegovy’s popularity waxed, its supply waned, and, encouraged by influencers, people turned to Ozempic for off-label weight loss (Ozempic’s active ingredient is also semaglutide, but it is only approved by the FDA for treating type 2 diabetes). This, in turn, caused shortages for people taking Ozempic to manage their type 2 diabetes.
A Refresher on Type 2 Diabetes
According to the Centers for Disease Control and Prevention (CDC), nearly 1 in 10 Americans have diabetes and about 90–95% of those have type 2 diabetes. Type 2 diabetes is a metabolic disorder where the body is unable to properly produce and respond to insulin, resulting in elevated blood glucose levels that can damage other bodily functions.
Blood glucose levels are regulated by a fine-tuned interplay of multiple body systems, signaling agents and nutrient intake (6). Briefly, when food and nutrients enter the small intestine, insulin is released from beta cells in the pancreas. Insulin promotes glucose uptake into tissues from the blood and stimulates glycogen formation from glucose. The overall impact is lowered blood sugar.
The underlying causes of type 2 diabetes are not explicitly known, but risk factors include a mix of genetics, obesity, low physical activity and diet.
Role of GLP-1 and GLP-1 Receptor Agonists in Regulating Insulin
Metformin (a small molecule guanidine derivative) is recommended as the first prescribed medication for managing patients’ type 2 diabetes (2). However, GLP-1 receptor agonists (like semaglutide) are often prescribed if metformin fails to properly regulate patients’ blood glucose levels and are designed to mimic glucagon-like protein-1 (GLP-1).
Glucagon-like peptide-1 (GLP-1) is a hormonal peptide that is released from L cells in the small intestine in the presence of nutrients (7). One of its many physiological roles is to mediate glucose-dependent insulin secretion by activating the GLP-1 receptor.
The GLP-1 receptor is a transmembrane protein belonging to the B class of G-protein coupled receptors and is expressed in beta cells in the pancreas (among other locations). When activated upon binding of GLP-1, the coupled G proteins stimulate adenylate cyclase, increasing production of cyclic AMP (cAMP) from ATP. Increased cAMP stimulates several downstream effects, including cell membrane depolarization and an increase in cytosolic Ca2+ concentration, that ultimately lead to secretion of insulin granules from beta cells (7).
Another way GLP-1 reduces blood sugar is by reducing glucagon release from pancreas alpha cells (8).
A key advantage of synthetic GLP-1 mimics is that they are long-lasting and designed to be resistant to degradation mechanisms (1). For example, native GLP-1 has a circulating half-life of only 1–2 minutes. Semaglutide, in contrast, has a circulating half-life of approximately one week. This extended half-life arises from amino acid swaps and an added fatty acid chain that make it more stable to degradation pathways (9).
GLP-1R Agonists and Weight Loss
The weight loss impacts of GLP-1 receptor agonists likely arise from several physiological effects (1). Firstly, activation of GLP-1 receptor slows stomach emptying into the intestine, which increases feelings of fullness during meals. The GLP-1 receptor is also expressed in the hypothalamus and studies suggest it helps regulate appetite. Overall, GLP-1 receptor agonists’ weight loss effects seem to arise from reducing people’s food intake.
Future Directions
One area of active research is to develop a small molecule GLP-1 receptor agonist (10), which would potentially be less expensive and more easily administered in a pill form than the current peptide-based therapies.
Additionally, peptides that act as agonists for GLP-1 receptor, the glucagon receptor and/or glucose-dependent insulinotropic polypeptide (GIP) receptor are showing promise for both managing type 2 diabetes and weight loss (10). Tirzepatide, for example, is a dual GLP-1/GIP receptor agonist that was recently approved for treatment of type 2 diabetes. Clinical trial results suggest that tirzepatide outperforms semaglutide, a sole GLP-1 receptor agonist, for controlling blood sugar and reducing weight.
Interested in learning more about type 2 diabetes and researching metabolic disorders? Check out this blog about a researcher who is investigating the genetic basis of type 2 diabetes or this infographic about assays for measuring insulin activity in cell models.
Does your research require an understanding of the metabolic profile of your cellular model? We have a portfolio of energy metabolism assays that can help.
References
- Nauck, M.A. et al. (2021) GLP-1 Receoptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol Metab. 46, 101102. doi: 10.1016/j.molmet.2020.101102
- Prasad-Reddy, L. and Isaacs, D. (2015) A Clinical Review of GLP-1 Receptor Agonists: Efficacy and Safety in Diabetes and Beyond. Drugs Context 4, 212283. doi: 10.7573/dic.212283
- Wharton, S. et al (2023) Two-Year Effect of Semaglutide 2.4 mg on Control of Eating in Adults with Overweight/Obesity: STEP 5. Obesity 31, 703. doi: 10.1002/oby.23673
- Rubino, D. M. et al. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes. J. Am. Med. Assoc. 327, 138. doi: 10.1001/jama.2021.23619
- Hinnen, D. (2017) Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 30, 202. doi: 10.2337/ds16-0026
- Saltiel, A. R. (2021) Insulin signaling in health and disease. J. Clin. Invest. doi: 10.1172/JCI142241
- Kuhre, R. E. et al. (2021) What is an L-Cell and How Do We Study the Secretory Mechanisms of the L-Cell? Front. Endocrinol. 12, 694284. doi: 10.3389/fendo.2021.694284
- Holter, M. M. et al. (2022) Alpha-Cell Paracrine Signaling in the Regulation of Beta-Cell Insulin Secretion. Front. Endocrin. 13, 934775. doi: 10.3389/fendo.2022.934775
- Lau, J. et al. (2015) Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide. J. Med. Chem. 58, 7370. doi: 10.1021/acs.jmedchem.5b00726
- Drucker, D. J. (2018) Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 27, 740. doi: 10.1016/j.cmet.2018.03.001
Related Posts
Latest posts by Jordan Nutting (see all)
- The Central Dogma of Promega: The Story and Science Behind Our Kit Packaging Design - May 7, 2024
- Silencing the Immunogenicity of AAV Vectors - April 4, 2024
- Discovering Cyclic Peptides with a “One-Pot” Synthesis and Screening Method - February 29, 2024

4 comments