A new study, published in the Journal of Molecular Diagnostics (1), highlights the potential of using long mononucleotide repeat (LMR) markers for characterizing microsatellite instability (MSI) in several tumor types. The paper is a result of a collaborative effort between researchers from Johns Hopkins University and Promega to evaluate the performance of a panel of novel LMR markers for determining MSI status of colorectal, endometrial and prostate tumor samples.
Microsatellite instability (MSI) is the accumulation of insertion or deletion errors at microsatellites, which are short tandem repeats of DNA sequences found throughout the genome. MSI in cancerous cells is the result of a functional deficiency within one or more major DNA mismatch repair proteins (dMMR). PCR-based MSI testing is a commonly used method that can help understand a tumor’s genomic profile as it relates to MMR protein function.
Historically, MSI has been a biomarker associated with Lynch syndrome, the hereditary predisposition to colorectal and certain other cancers. In recent years, research interest in MSI has exploded, driven by the discovery that its presence in tumor tissue can be predictive of a positive response to anti-PD-1 immunotherapies (2,3).
Why MSI is Useful with Immunotherapies
When mistakes in DNA replication go uncorrected in cancer cells, they produce “foreign” proteins, called mutation-associated neoantigens (MANA), which can be detected by the immune system. Tumors expressing these proteins are effective at priming an immune response and subsequently susceptible to immunotherapies such as immune checkpoint inhibitor therapies. In tumors, MSI-High (MSI-H), as determined by PCR-based assays, is a recognized biomarker for these therapies. In clinical research, the current industry standard for PCR-based MSI testing is the Promega MSI Analysis System, Version 1.2 (MSI Analysis System), which uses a panel of five mononucleotide repeats, each composed of 21–27 repeated adenine bases (4).
Evaluating Long Mononucleotide Repeat Markers for Detecting MSI
Long mononucleotide repeat (LMR) markers tend to be more unstable and, when amplified, the unrepaired replication errors in these markers will often result in larger base pair shifts. This could make LMR markers more sensitive at detecting MSI and prove especially useful in samples where current tests for dMMR or MSI return inconclusive results. The LMR MSI Analysis System (Promega) offers an eight-marker panel that contains four of the gold standard markers from the MSI Analysis System and four LMR markers with repeat lengths of 52–60 adenine bases.
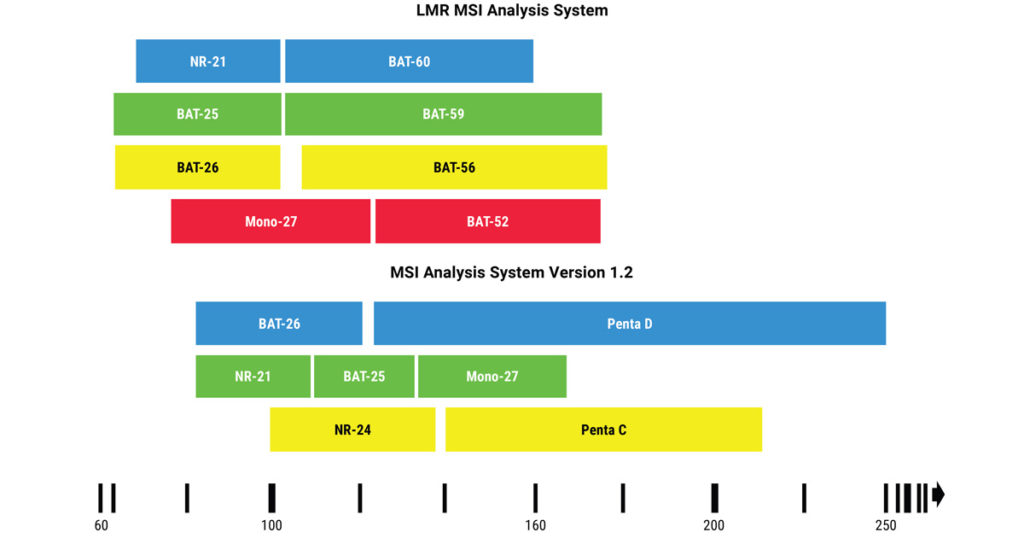
In the study published in the Journal of Molecular Diagnostics (1), the authors validated the sensitivity and specificity of the LMR panel for characterizing MSI in colorectal, endometrial and prostate tumor samples using immunohistochemical (IHC) determination of dMMR and/or MSI results from the MSI Analysis System as comparator methods. The study also included 22 tumor samples from other cancer types that had been designated MSI-H as well as a small set of tumors that had returned MSI-Low (MSI-L) results with previous MSI testing.
In the colorectal cancer tumor sample set evaluated, the LMR panel showed 100% concordance with the MSI Analysis System, identifying all 24 samples known to be dMMR by both IHC and the MSI Analysis System (100% sensitivity and specificity). In endometrial tumor samples, the LMR panel showed better sensitivity compared to the panel of shorter markers, identifying 41 out of 42 samples known by IHC to be dMMR (98% sensitivity and 100% specificity). For the 12 dMMR prostate cancer samples included in the study, the LMR panel identified nine as MSI-H (75% sensitivity). In both the prostate and endometrial tumor samples, the LMR markers produced larger shifts in base size compared to the shorter repeat markers (7.4–11.2 vs. 3.7–4.8 and 6.0–12.8 vs. 2.1–4.2, respectively). These larger shifts helped improve sensitivity in these sample types.
In the set of 22 samples from other cancer types, the LMR panel showed 100% concordance with the previous MSI-H designation determined using the MSI Analysis System. For certain cancer types within this set, some LMR markers displayed larger shifts compared to the shorter markers. These results are similar to those observed in the endometrial and prostate cancer sets. Of the 12 tumor samples that had tested MSI-L with the MSI Analysis System, the LMR panel was able to clarify results for four samples, indicating one sample was MSI-stable (MSS) and three were MSI-H. These results suggest that LMR markers could be useful for confirming MSI results in some cancers as well as for clarifying MSI status in tumors that produce inconclusive or ambiguous MSI results with other markers or testing methods.
LMR Markers Show Promise for MSI Characterization
This validation study demonstrated the promise and utility of LMR markers for adding sensitivity and clarity to MSI testing among certain cancer types. The LMR marker panel allows researchers to achieve an expanded view of the level of instability present in their samples and improve their ability to characterize MSI.
References
- Lin, J.H. et al. (2022) J. Mol. Diagn. 24, 144–57.
- Le, D.T. et al. (2015) N. England J Med. 372, 2509–20.
- Dudley, J.C. et al. (2015) Clin. Cancer Res. 22, 813–20.
- Bacher, J.W. et al. (2004) Dis. Markers 20, 237–50.
Learn more about the LMR MSI Analysis System
Here is more about MSI testing solutions for cancer research
Looking for more information about MSI? Check out the MSI Resource Center.
