 Anyone who has travelled across time zones knows how unpleasant it is when the regular rhythm of your biological clock is disrupted. Jetlag results when the body’s internal clock, or circadian rhythm is out of sync with external cues for “day and “night”, resulting in insomnia, extreme tiredness, difficulty concentrating and various other unpleasant symptoms.
Anyone who has travelled across time zones knows how unpleasant it is when the regular rhythm of your biological clock is disrupted. Jetlag results when the body’s internal clock, or circadian rhythm is out of sync with external cues for “day and “night”, resulting in insomnia, extreme tiredness, difficulty concentrating and various other unpleasant symptoms.
On the bright side, jetlag is at least a temporary misery that is usually over after a few days of acclimation to the new time zone. Long-term disruption of the natural sleep/wake cycle, such as encountered by frequent long-distance travellers, shift workers, or people with physiological conditions that affect circadian rhythms, can be much more debilitating. Longer term health effects that have been associated with constant disruption of circadian rhythms include, insomnia, concentration problems, and increased susceptibility to diseases associated with chronic inflammation such as cancer, diabetes and cardiovascular disease.
Despite the fact that many of the genes and proteins involved in central control of circadian rhythms are known, the reason for the implied association between circadian clock components and immune function is not understood. Recently, a paper was published in the July issue of PNAS that identified a potential link between a circadian clock component and chronic inflammation.
Of Flies, Mice and Men
The molecular mechanisms controlling circadian rhythms are well conserved between species, and the core clock genes and proteins have been described in fruit flies and mice. The genes first characterized in fruit flies have homologs of similar function in mammalian systems. This core molecular clock orchestrates a large number of physiological processes, including hormonal activity and metabolic activity, in accordance with a sleep/wake cycle of around 24 hours in humans. Because the period is not exactly 24 hours, it needs to adjust daily by responding to environmental signals known as Zeitgebers (time-givers), one of the main signals being light.
Mammalian circadian rhythms are controlled by a master clock located in the brain (in the hypothalamic suprachiasmatic nuclei). The period of biological rhythm (day) is the result of a complex interplay between core clock proteins, environmental signals, and peripheral circadian oscillator proteins in other cells throughout the body, much of which is not yet fully understood. The transcriptional activators CLOCK and BMAL together bind to and activate production of the PERIOD and CRYPTOCHROME genes, which in turn bind to each other and inhibit their own production by preventing binding of CLOCK and BMAL to the promoter region, effectively slowing the cycle at the end of the day. In the presence of the zeitgeber signal, the biological clock adjusts its period to the environmental cycle, and the ensuing cascade of time-synchronized biological activities (metabolism/hunger/hormones/sleep, etc.), align to the environmental cues. Hence the discomfort when we try to force our bodies to adjust to a new schedule in the absence of the correct environmental cues. For example, travelling east and “missing” the normal signals for dawn and dusk, or making an abrupt transition to a night shift.
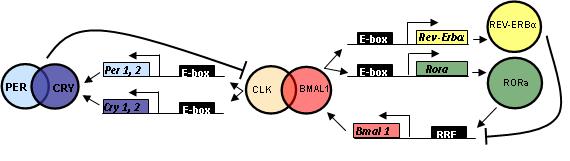
An Immune Connection
Even a cursory glance at the literature on this subject reveals the tremendous range and complexity of the biological processes and molecular relationships that are affected by circadian signals. The complex molecular relationships between these processes are only beginning to be worked out. The July PNAS paper has identified a possible molecular link between circadian rhythm disturbances and increased inflammatory response for the first time. The paper identified a potential connection between the key circadian clock component Cryptochrome and regulation of immune function, identifying a molecular reason for the link between chronic sleep deprivation, circadian regulation and inflammation. The authors found that mice lacking the circadian oscillator component Cryptochrome (CRY) had high levels of proinflammatory cytokines and showed increased NF-κB and protein kinase A activation. Since Cryptochrome inhibits CLOCK-BMAL activation of the PERIOD and CRY genes, effectively slowing the system at the end of the day, the researchers suggest that reduced CRY expression in chronic sleep deprivation may have a direct role in generating the cellular stress that causes persistant, low level inflammation.
The identification of this link is significant in that it pinpoints one potential reason for the observed association of susceptibility to inflammatory disease with circadian rhythm disruption.
Here’s the Paper
![]() Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, & Verma IM (2012). Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America, 109 (31), 12662-7 PMID: 22778400
Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, & Verma IM (2012). Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proceedings of the National Academy of Sciences of the United States of America, 109 (31), 12662-7 PMID: 22778400
More on this Story
Science Daily Report
Isobel Maciver
Latest posts by Isobel Maciver (see all)
- 3D Cell Culture Models: Challenges for Cell-Based Assays - August 12, 2021
- Measuring Changing Metabolism in Cancer Cells - May 4, 2021
- A Quick Method for A Tailing PCR Products - July 8, 2019
