Remdesivir (RDV or GS-5734) was used in the treatment of the first case of the SARS-CoV-2 (formerly 2019-nCoV ) in the United States (1). RDV is not an approved drug in any country but has been requested by a number of agencies worldwide to help combat the SARS-CoV-2 virus (2). RDV is an adenine nucleotide monophosphate analog demonstrated to inhibit Ebola virus replication (3). RDV is bioactivated to the triphosphate form within cells and acts as an alternative substrate for the replication-necessary RNA dependent RNA polymerase (RdRp). Incorporation of the analog results in early termination of the primer extension product resulting in the inhibition.
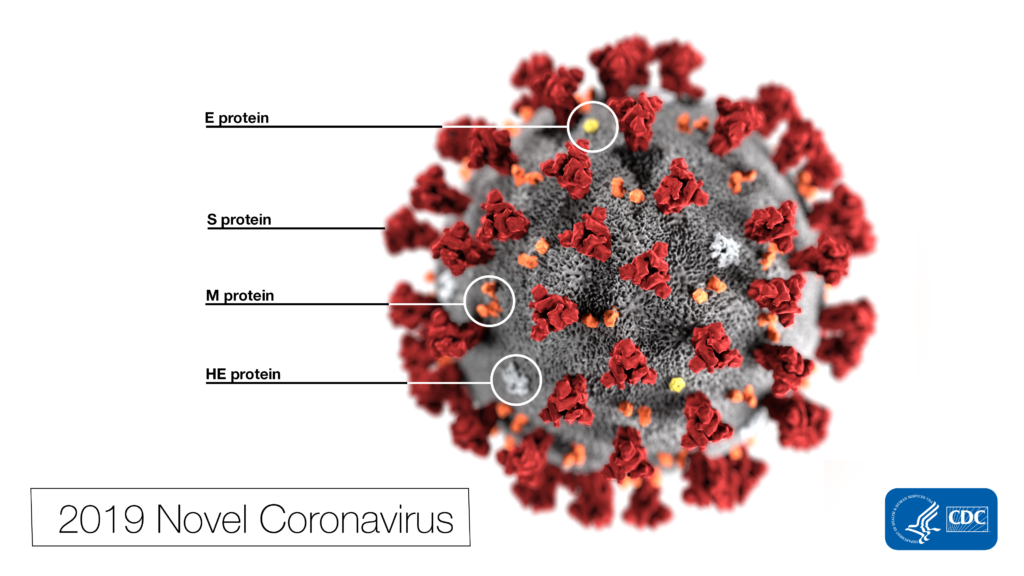
Why all the interest in RDV as a treatment for SARS-CoV-2 ? Much of the interest in RDV is due to a series of studies performed by collaborating groups at the University of North Carolina Chapel Hill (Ralph S. Baric’s lab) and Vanderbilit University Medical Center (Mark R. Denison’s lab) in collaboration with Gilead Sciences.
Coronavirus replication is dependent upon a viral RdRp as well for replication. Sheahan, et al. (4) decided to examine the effect of RDV on coronavirus replication despite an earlier report (5) of the core nucleoside of the RDV, GS-441524, being ineffective against the SARS coronavirus. Sheahan and colleagues developed NanoLuc® Luciferase (NLuc)-tagged SARS and MERS coronaviruses for a rapid assessment of whether RDV could inhibit replication. Studies in the human lung epithelial cell line, Calu-3 2B4, demonstrated that the compound inhibited MERS-NLuc virus replication with an IC50 of 0.03µM when cells were challenged with 0.08 MOI of virus over a 48 hour period. The compound alone did not demonstrate cytotoxicity to the Calu cells in tests with up to 10µM RDV as judged by a CellTiter-Glo® Assay. Further work demonstrated a dose-dependent decrease in viral titer and viral RNA for both SARS and MERS CoV in human airway epithelial cell culture as judged by RT-qPCR. RDV efficacy was also demonstrated in a mouse model of SARS CoV infection. A key point about using RDV was that it needed to be administered either before or shortly after infection to limit lung damage. Administration at peak viral titer could not correct lung damage that had already occurred.
Learn more about NanoLuc® Reporter Viruses at our website.
A current clinical trial of a possible treatment for MERS-CoV is currently underway in Saudi Arabia using a combination lopinavir, ritonavir and interferon-β (LRI; 6). Sheahan, et al. (7) investigated how RDV would perform in comparison to the trial treatment. Using a NLuc-tagged MERS-CoV showing almost identical susceptibility to RDV as wild-type virus, the treatments were evaluated in a cell model and RDV proved more effective than the separate trial compounds or the combination of all three trial compounds. The lab developed a mouse model for analysis of MERS-CoV infection and found that pre-infection and early infection treatment of the mice with RDV reduced lung viral loads and severe lung pathology. In contrast, pre-infection treatment with LRI slightly reduced viral loads without impacting other disease parameters. Post-infection LRI treatment did not reduce viral loads or sever lung pathology.
Agostini, et al (8) examined the mechanism of how RDV inhibited CoV replication. The CoV genome codes for an exoribonuclease (ExoN) as well as the RdRp. The ExoN serves a proofreading function and previous work in the lab demonstrated that the ExoN activity prevents sensitivity to mutagens like like 5-fluoruracil and ribavarin (9). The lab chose a model coronavirus, mouse hepatitis virus (MHV), to investigate the ExoN function in CoV susceptibility to RDV. Treatment with RDV or the parent nucleoside GS-441524 reduced MHV viral titers in a dose-dependent manner with the RDV being more than 30-fold more potent. Similar results were obtained with SARS-CoV and MERS-CoV. Knock out of the ExoN resulted in a 100-fold increased sensitivity to RDV suggesting that the wildtype MHV, despite a functional ExoN system, still incorporated RDV into the viral RNA. Like with the SARS-CoV study earlier, timing of RDV dosage is important. In a cellular model, administration of remdesivir between -2 to +2 hours post-infection severly limits viral replication.
Learn how Promega is supporting CROs providing services for COVID-19 testing and drug discovery.
A concern with any virus is the potential of mutations producing treatment-resistant viruses. MHV was passaged in the presence of GS-441524 and one isolate was found to survive past 23 passages. Sequencing of the genome revealed 6 mutations most notably two within the RdRp of MHV. These two residues were conserved in many CoV genomes including SARS and MERS. Introduction of the two mutations into wildtype MHV resulted in a virus with nearly identical characteristics to the passage 23 MHV. Both the double mutant MHV and p23 MHV were still susceptible to RDV, just requiring higher doses. The double mutant MHV was a less fit virus as co-infection studies with wildtype virus at different ratios yielded almost only wildtype virus after four passages. Introduction of these two mutations into the SARS CoV yielded much the same story in that the double mutant was more resistant to RDV. This study was performed with NLuc-tagged wildtype and double mutant SARS-CoV. Using the mouse model system described earlier (4), the double mutant was less pathogenic than the wildtype virus.
A recent letter to the editor by Wang, et al. (10) reports that RDV is the most effective in vitro inhibitor of clinical isolates of SARS-CoV-2 among a panel of antivirals tested. Interestingly, they also observed substantial inhibition by the anti-malarial drug, chloroquine. Chloroquine is known to inhibit acidification of endosomes and may interfere with internalization of the virus.
References:
- Holshue, M.L., et al. (2020) First case of 2019 novel coronavirus in the United States. New Eng. J. Med. DOI: 10.1056/NEJMoa2001191
- “Gilead Sciences Statement on the Company’s Ongoing response to the 2019 Novel Coronavirus (2019-nCoV),” Gilead Sciences, 31 January 2020. [Online]. Available: . [Accessed 12 February 2010]
- Warren, T.K., et al. (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 431, 381–5.
- Sheahan, T.P., et al. (2017) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653.
- Cho, A., et al. (2012) Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 22, 2705–7.
- Arabi, Y.M., et al. (2018) Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 19, 81.
- Sheahan, T.P., et al. (2020) Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Comm. 11, 222.
- Agostini, M.L., et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 9, e00221–18.
- Smith, E.C., et al. (2013) Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 9, e1003565.
- Wang, M., et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. DOI: 10.1038/s41422-020-0282-0
Ralph S. Baric, PhD link: https://sph.unc.edu/adv_profile/ralph-s-baric-phd/
Mark R. Dennison, MD link: https://www.vumc.org/denison-lab/laboratory-mark-denison-md
