This post was written by guest blogger, Nicole Werner, Product Management Support at Promega GmbH.
“You have cancer.” – a statement that fundamentally changes life in a second. After the first shock, the insight often arises: “If only I had stopped smoking sooner!”
Lung cancer, while not the leading cause of death worldwide, is the leading preventable cause of death in developed countries. According to the WHO, eight million people die each year as a result of smoking, including one million as a result of passive smoking [1]. Currently, 80% of those affected die within the next 13 months after diagnosis [1]. New therapeutic approaches, such as treatment with immune checkpoint inhibitors, bring hope.
Promega supports research in this area with the high-precision tools needed to develop this new form of therapy.
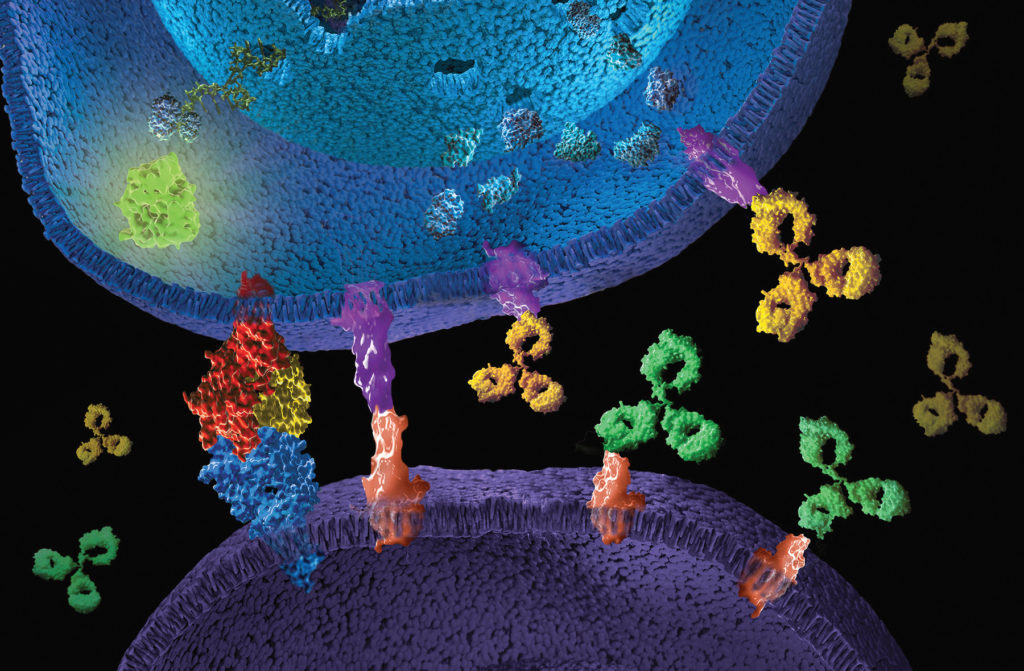
What are immune checkpoint inhibitors?
Immune checkpoints are part of our immune system and serve a targeted regulation of the immune response. They act as receptor proteins on the surface of T cells that recognize partner proteins on other cells and bind to them. When the checkpoint and partner proteins come together, they send a specific retraction signal to the T cells. This prevents the immune system from destroying healthy cells in the body. However, some cancer cells use this, (e.g. through increased expression of the checkpoint partner proteins), to bypass an immune attack. Immune checkpoint inhibitors (ICI) are mostly therapeutic monoclonal antibodies that are directed against specific checkpoint proteins, thereby restoring or enhancing the body’s own immune response against the cancer cells, leading to their selective killing.
With the approval of the first ICI drug (ipilimumab, a CTLA-4 inhibitor) 10 years ago, this form of treatment quickly developed into the fourth pillar of cancer therapy [2] and is used as a single agent or in combination with chemotherapy as a first or second line drug [3]. This offers new treatment options for poorly treatable tumors such as black skin cancer or lung cancer. Seven checkpoint inhibitors are currently available in Europe: nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, cemiplimab, and ipilimumab [4]. Studies with nivolumab in combination with ipilimumab show a significant improvement in life expectancy compared to conventional chemotherapy for metastatic non-small cell lung cancer [5]. However, undesirable side effects due to an unexpected immune response can also occur with ICI therapy [3,5]. Research therefore focuses intensively on elucidating the mechanisms of action, with the aim of improving therapies and reducing side effects. Due to the biological complexity of therapeutic antibodies, quantitative and reproducible analytical tools are required.
Mechanism-of-Action Based Immune Checkpoint Bioassays for Research and Characterizing Therapeutic Antibodies
Promega supports oncologists, cell biologists and immunologists in their fight against cancer. A broad portfolio of functional reporter cell lines and bioassays is available to quantitatively measure the effectiveness of therapeutic antibodies directed against immune checkpoint proteins. The checkpoints act either co-inhibitory, such as the receptors PD-1, CTLA-4, LAG-3, SIRPα with their respective binding partners, or also co-stimulatory, such as 4-1BB, GITR, ICOS with their partners T cell response. These bioassays consist of stable cell lines that express a luciferase under the control of a receptor-mediated intracellular signal. In the following poster presentation (PDF of poster), Jamison Grailer, Senior Research Scientist at Promega, describes the application of these MOA (Mechanism-of-Action) -based bioassays for development, screening, potency and stability studies of therapeutic antibodies.
Immune checkpoint bioassays are only a small part of the Promega portfolio for the characterization and development of new monoclonal antibodies or cell-based therapeutics. We have a dynamic portfolio of quantitative, reproducible, and high-precision bioassays for the investigation of Fc effector activity, immune checkpoint modulation, T-cell activation as well as cytokine and growth factor signaling and are already working with research partners from the pharmaceutical and academic sectors together in the early stages of assay development to ensure that our bioassays meet their needs.
A New Perspective on Cancer Treatment
ICI therapy has revolutionized cancer research and fundamentally changed the way we view cancer treatment. In treatment, cancer cells are no longer the only focus, but also their immunological environment, which leads to a more holistic view of the patient and will enable personalized therapy in the future.
Are you interested in a partnership? Do you have questions about our bioassays and cell lines? Please contact us. You can find more information in our bioassay brochure .
Literature Cited
- WHO Global Report on Trends in Prevalence of Tobacco Use 2000-2025, third edition. 2019. Geneva: World Health Organization.
- McCune, J.S. (2018) Rapid Advances in Immunotherapy to Treat Cancer. Clin. Pharmacol. Ther. 103(4):540-544. https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1002/cpt.985
- Robert, C. (2020) A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11, 3801. https://doi.org/10.1038/s41467-020-17670-y
- Ruggiero, S. et al. (2020) Immune Checkpoint Inhibitors and Immune-Related Adverse Drug Reactions: Data From Italian Pharmacovigilance Database. Front Pharmacol 11, 830. https://doi.org/10.3389/fphar.2020.00830
- Paz-Ares L, et al. (2021) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22(2):198-211. https://pubmed.ncbi.nlm.nih.gov/33476593/
Related Posts
Latest posts by Promega (see all)
- Immune Surveillance Meets Innovation: The Critical Need for dsRNA Detection - April 22, 2025
- Beyond Ozempic: The New Frontier of Obesity Research - April 18, 2025
- One Health and H5N1: Promega’s Commitment to Holistic Solutions - April 8, 2025
