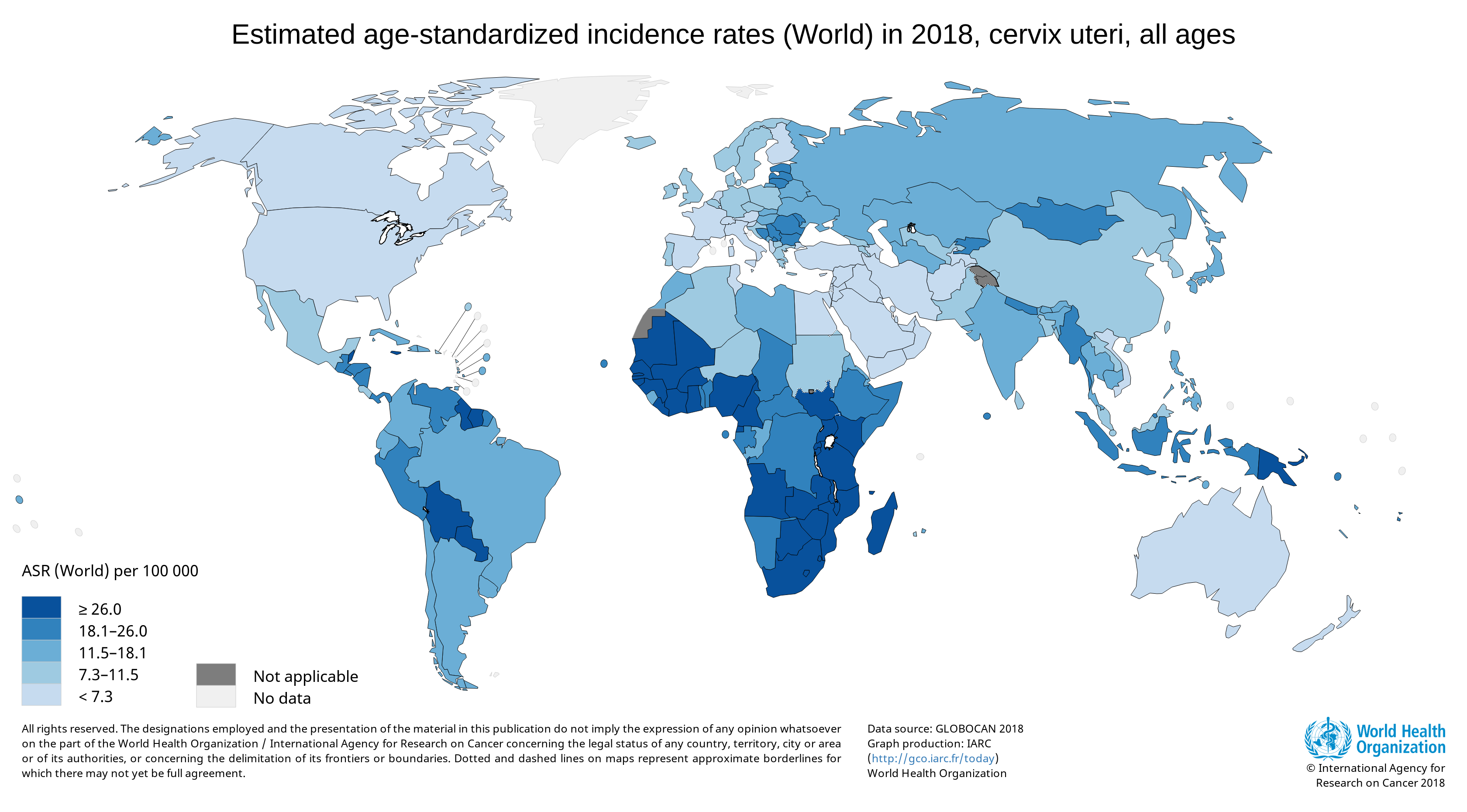
Cervical cancer is a major health problem for women, and it is currently the fourth most common cancer in women globally (1). A worldwide analysis of cancer estimates from the Global Cancer Observatory 2018 database showed that cervical cancer disproportionally affects lower-resource countries, on the basis of their Human Development Index; it was the leading cause of cancer-related death in women in many African countries (1).
Infection by human papillomavirus (HPV), a double-stranded DNA virus, is the leading cause of cervical cancer. Many types of HPV have been identified, and at least 14 high-risk HPV types are cancer-causing, according to a World Health Organization (WHO) fact sheet. Of these types, HPV-16 and HPV-18 are responsible for 70% of cervical cancers and pre-cancerous cervical lesions. HPV infection is sexually transmitted, most commonly by skin-to-skin genital contact. Although the majority of HPV infections are benign and resolve within a year or two, persistent infection in women, together with other risk factors, can lead to the development of cervical cancer [reviewed in (2)].
It is unclear why most HPV infections do not lead to the development of cervical cancer. Research has shown that the initial mechanism of transformation is characterized by the deregulated expression of viral E6 and E7 genes in the basal, proliferating cells of the epithelium. In transformed host epithelial cells, aberrant expression of host genes is cumulative, resulting in eventual progression to cancer—a process that can take 20–30 years (3). Epigenetic alterations in specific genes have been detected during this process, and clinically validated panels of methylation markers can be used as triage markers for HPV-positive women (3). Many of the genes that are dysregulated during the progression to cervical cancer encode microRNAs (miRNAs): small, noncoding RNA molecules that regulate the expression of specific messenger RNA (mRNA) targets (4).
A recent study examined the combination of changes in DNA copy number, and miRNA and mRNA expression, that occur over time as a result of HPV transformation (5). The study used four individual HPV-transformed keratinocyte cell lines at eight different passages, representing different stages of HPV-induced transformation.
Due to the complexity of the molecular changes involved during HPV transformation and subsequent progression to cervical cancer, it has been difficult to identify potential therapeutic targets. Many studies have focused on differential gene expression patterns. However, as Dr. Saskia Wilting, the corresponding author of the study explains, “Differential gene expression studies yield high numbers of altered genes. In general, it is quite difficult to determine which genes are truly driving the carcinogenic process and which genes are so-called passengers.” The “passenger” genes exhibit altered expression patterns during the development of carcinogenesis, but they are not directly relevant to the progression of disease.
To identify genes relevant to HPV-induced carcinogenesis, the researchers performed integrative longitudinal differential gene expression analysis, which also takes the genomic copy number into account. They identified 3,642 mRNA genes and 106 mature miRNAs whose expression was altered over time; of these, approximately one-third showed an association with copy number. Interestingly, three of the most significantly overrepresented pathways among all DNA copy number-associated genes were implicated in development of anchorage independence—a characteristic of complete transformation in vitro. These pathways were focal adhesion, TGF-beta and mTOR signaling.
Paradoxically, TGFB1 is known to act as a tumor suppressor during early stages of cervical carcinogenesis, although it takes on a tumor-promoting function at a later stage. The researchers identified the paired-like homeodomain 2 (PITX2) protein as a key regulator of TGF-beta signaling and examined its functional role by overexpression experiments.
“We hypothesize that downregulation of PITX2 during HPV-induced transformation may result in a different effect of TGF-beta signaling,” Dr. Wilting says. She further elaborated on a potential explanation for the apparent paradox seen for TGFB1 activity. “Early in the process, PITX2 is still present and TGF-beta signaling appears to have a tumor-suppressive effect. Further in the transformation process, PITX2 becomes downregulated and TGF-beta signaling has an oncogenic effect.”
Next, the researchers investigated miRNA-mRNA expression patterns thought to be altered following transformation, using mRNA expression analysis and the Dual-Glo® Luciferase Assay System. They confirmed miRNA-mRNA binding in transient cotransfection experiments of the miRNA gene with either a wild-type or mutant predicted target mRNA sequence. Dr. Wilting cited the convenience of measuring the Renilla luciferase internal control activity in the same assay as the experimental firefly luciferase signal. “In general,” she says, “variation within and between experiments seems to be less, due to the incorporation of the control luciferase signal in the same construct.”
Subsequent experiments identified two miRNAs (miR-30a-3p and miR-138-5p) whose ectopic expression decreased anchorage-independent growth of late-passage FK18B cells. No reduction in cell viability was observed on normal, adherent plates, suggesting involvement of these miRNAs specifically in anchorage independence.
Future research will identify the precise roles of these miRNAs in the development of cervical cancer. For example, Dr. Wilting hopes to “experimentally alter the expression of the identified target mRNAs both in early and late passages of our cell lines, and determine whether this would change the phenotype of the cells accordingly.”
Learn about a newer, more sensitive dual-luciferase assay: the Nano-Glo® Dual-Luciferase Reporter Assay.
References
- Arbyn, M. et al. (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob. Health. 8(2), e191–e203.
- Burd, E.M. (2003) Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 16(1), 1–17.
- Steenbergen, R.D.M. et al. (2014) Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nature Rev. Cancer 14, 395–405.
- Pardini, B. et al. (2018) MicroRNAs as markers of progression in cervical cancer: a systematic review. BMC Cancer (2018) 18, 696.
- Babion, I. et al. (2020) Identification of deregulated pathways, key regulators, and novel miRNA-mRNA interactions in HPV-mediated transformation. Cancers 12, 700.
Related Posts
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023
