
Malaria affects nearly half of the world’s population, with almost 80% of cases in sub-Saharan Africa and India. While there have been many strides in education and prevention campaigns over the last 30 years, there were over 200 million cases documented in 2017 with over 400,000 deaths, and the majority were young children. Despite being preventable and treatable, malaria continues to thrive in areas that are high risk for transmission. Recently, clinicians started rolling out use of the first approved vaccine, though clinical trials showed it is only about 30% effective. Meanwhile, researchers must continue to focus on innovative efforts to improve diagnostics, treatment and prevention to reduce the burden in these areas.
The circle of life
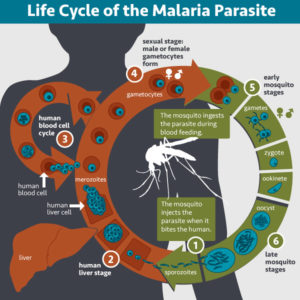
Eradicating malaria is difficult partially because the transmission cycle is complex. The parasites of the genus Plasmodium are transmitted by a mosquito vector. When a mosquito infects a human host, the multiplication and maturation of the parasite results in disease. Symptoms can be mild—fever, headaches, nausea, vomiting/diarrhea—similar to other flu-like illnesses. However, if left untreated, severe illness can occur, such as anemia, organ failure, seizures, coma, and death.
The parasite can infect a new mosquito when it matures to its sexual stages of development. When a mosquito feeds on infected human blood, the internalized parasite transforms into male and female gametes that fertilize and form a zygote. The zygote matures through several forms in the fertilization stage before replicating into thousands of copies of its infectious form, called sporozoites. At this stage in the Plasmodium life cycle, the sporozoites migrate to the mosquito salivary glands, where they are transmitted into a new human host upon the mosquito’s next blood meal.
Recent studies to genetically modify mosquitoes demonstrate the ability reduce the vector population and control malaria transmission. However, gene editing remains a controversial technology. As scientists and ethicists weigh the pros and the cons, there are other efforts to prevent transmission through novel drug discovery.
The very model of a transmission-blocking drug compound
Many known transmission-blocking compounds inhibit or kill the parasite in its sexual stage of development (gametocytes), but there has been little research to identification of compounds that target Plasmodium gametes that are formed early within the mosquito vector. A recent study describes a novel screening model to identify drug compounds that block transmission during the fertilization stages.
Calit et al. created a transgenic parasite model using the species Plasmodium berghei combined with NanoLuc® luciferase. Since luciferase expression and activity in this mutant strain is controlled by the ookinete-specific promoter CTRP, the researchers named their transgenic line Ookluc. They tested this strain with an in vitro conversion assay. When the parasite was in the circulating blood stages, there was little luminescence. When the parasite converted into mosquito sexual stages, luminescence increased, suggesting that NanoLuc® expression in Ookluc is dependent on gamete development.
Next, the Ookluc strain was used to screen for compounds in the Pathogen Box from the Medicines for Malaria Venture. This research kit contains 400 compounds that are active against neglected tropical diseases—125 of the compounds are known to target malaria. In the assay, 31 compounds inhibited over 95% of Ookluc conversion into the fertilization stage. Nine of those compounds were also effective at inhibiting Ookluc conversion at a low concentration.
Due to the high sensitivity of NanoLuc® luciferase, the Ookluc model is ideal for high-throughput screening of transmission-blocking compounds by targeting the life cycle of the parasite. Though, identification of these therapeutic drugs is just one part of the solutions needed for total eradication of malaria.
Crease on down the fold
Given that most malaria cases are in low-resource areas, there is a strong need for low-cost, reliable tools for diagnosing disease. PCR is the “gold standard” diagnostic method, but access to lab equipment and trained staff are limitations for use in these regions. Light microscopy is a common technique currently being used, requiring only a microscope and a technician. However, this technique has limitations for diagnosing asymptomatic disease. “There is a need to be able to detect infected patients who do not show strong symptoms and can serve as a reservoir for the disease,” says Jonathan Cooper, a bioengineer from the University of Glasgow, and senior author on a recent paper offering a unique diagnostic solution.
Reboud et al. developed a paper-based microfluidic device for DNA extraction, amplification, and detection using just a few simple origami folds. “We aimed to provide a technique that could catch these low infected patients, and yet be used in the field—with no training,” says Cooper. The method is highly sensitive and fast—about 45 minutes—and able to detect malaria in 98% of infected individuals.
The paper folding technique allows for manipulation of sample fluid while keeping reagents separated until needed for processing. Only 5ul of blood is needed for the test, and it’s combined with magnetic MagaZorb® beads to bind the DNA. After washing, the paper strip is folded over and creates a distribution channel for DNA elution. This is then placed against the diagnostic device which has three loop-mediated isothermal amplification (LAMP) reaction chambers with paper-based valves to separate the reagents. “This is essential for molecular assays, as the sample contains inhibitors of the reactions used (the amplification) which need to be washed away,” says Cooper. “Valving is essentially bringing two things together at a particular time but not before, which is what folding enables us to do.” The LAMP assay amplifies DNA without temperature cycling and provides a visual diagnostic.
Using this method was comparable to real-time PCR results done in a laboratory setting. This suggests that cheap and rapid diagnostic tools can be made available for communities lacking technological infrastructure. The goal is to reduce the burden of disease by quickly diagnosing and treating those in need.
The impossible dream
Eradication of malaria seems like an impossible journey. However, malaria has been effectively eliminated from many countries thanks to education and prevention efforts. Total global elimination is possible, though the burden in rural communities is a barrier. There is much work to be done, but as scientists continue to think outside the box and develop innovative research strategies, there is hope that we can overcome these global challenges.
Latest posts by Mariel Mohns (see all)
- Sustainability Makeover: Parking Ramp Edition - January 27, 2020
- Go with Your Gut: Understanding How the Microbiome and Diet Influence Health - November 26, 2019
- Kyle Hill Explains the Science of Star Wars at the Wisconsin Science Festival - October 23, 2019
