 For many, this time of year brings with it the opportunity to enjoy a bit of holiday fun with kids. In fact just recently I had the chance to spend a day doing several home science activities with my four- and seven-year old boys. All were simple to set up using commonly found household items in a way that was both instructive and rewarding.
For many, this time of year brings with it the opportunity to enjoy a bit of holiday fun with kids. In fact just recently I had the chance to spend a day doing several home science activities with my four- and seven-year old boys. All were simple to set up using commonly found household items in a way that was both instructive and rewarding.
One of our introductory experiments had a chemistry flavor to it, designed as it was to show how acids can affect the color of tea (1). Tea leaves contain a group of chemicals known as polyphenols that give tea its characteristic taste and color. The change in color that is seen when lemon (acid) is added to the tea results from a reaction involving one specific group of polyphenols called thearubigins (1). When thearubigins are put into an acid environment they turn a lighter color. To demonstrate this, we dropped a tea bag in hot water and allowed it to brew for a few minutes. We then slowly squeezed in half a lemon drop-wise and observed how the tea turned from a dark to a pale brown.
Fresh from his discoveries of thearubigins, my oldest son caught on to the idea that science is not only about recording data but also about drawing conclusions on what the data reveals about the world around us. Temperature and pressure in particular are concepts that he often hears about while listening intently to local weather forecasts. In our second experiment we decided to put these two concepts to the test using two empty, tightly capped plastic bottles. We placed one of these in a freezer, left the other at room temperature and waited 30 minutes. When we investigated further we found that the bottle left in the freezer has collapsed in on itself.
With a little encouragement both my sons set out to unravel the mystery behind their collapsing frozen bottle. While what they found out surprised them, they dutifully reported back on their findings. Their conclusion? After being in the freezer the air in the bottle had cooled down. Cold air of course occupies less volume and exhibits a lower pressure than warm air. As the air had cooled, it had contracted causing the bottle walls to collapse. We were all later enthralled by the discovery that we could generate a decrease in air pressure and thereby produce a nice spray effect by simply holding one straw in a glass of water and blowing hard horizontally over the top of it with another (2). Innocent water-blowing fights inevitably became the order of the day from that moment on.
Still, our science antics were far from over. After vividly recalling how static electricity had exacerbated the dry air effects of the previous winter, we diligently set out to recreate some sparks of our own. We scrunched up a bin liner and rubbed it on a blanket for about 30 seconds (3). We then lightly turned on a bathroom faucet and held the bin liner ‘ball’ next to the running water. The effects of electrostatic attraction were plain to see as the stream of water bent towards the now-charged ball (3).
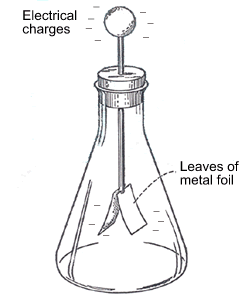
Eager to fulfill an inner craving for all things machine-related we finished off by building a simple static electricity ‘charge detector’ (otherwise known as an electroscope). A cardboard circle with a nail stuck through it holding two 1 × 1 cm pieces of aluminum foil mounted inside a glass jar was all it took to have our charge detector ready. A few strokes of a comb through our hair gave us the charge we needed to repel the aluminum foil pieces away from each other.
Tired and famished but intellectually satisfied, we turned in for dinner. And yet amidst the chatter over the day’s events, there was still one question that continued to engage my sons’ curiosity: “Daddy,” they asked, “what does a scientist really do all day?”. My answer was simple: “Research, my sons, research”. Needless to say, the ensuing conversation around the new ‘R word’ was anything but simple. But that my dear readers is the subject of another story.
Literature Cited
- New Scientist: Does Anything Eat Wasps? And 101 Other Questions (Questions and answers from the popular ‘Last Word’ column), Profile Books, London, UK, p.116
- Crazy Quarter and Marvelous Mist in Experiments You Can Do In Your Kitchen, Published by Waterbird Books, Columbus, Ohio, pp.29-33
- Bend Some Water in 101 Great Science Experiments, by Neil Ardley, DK Publishing, 1993, p.50
Robert Deyes
Latest posts by Robert Deyes (see all)
- Royal Lessons for Scientific Discovery - October 28, 2016
- Because Timing Is Everything…. - August 18, 2014
- Grays On the Move: Whale Watching in San Diego - April 30, 2014

Thank you Usakswar. I would be interested in knowing which parts of the experiments we did were of help to you.
Robert