 Researchers working with immortalized cell lines would readily agree when I state that it is almost impossible to look at cells under the microscope and identify them by name. There are phenotypic traits, however they do change with change in media composition, passage number and in response to growth factors. I remember the pretty arborizations my neuroblastoma cell line SH-SY5Y exhibited in response to nerve growth factor treatment. Thus physical appearance is not a distinguishing feature. Currently, in many labs, researchers typically use more than one cell line, and more than likely, share the same lab space to passage cells and the same incubator to grow the cells. In such scenarios, it is not difficult to imagine that cell lines might get mislabeled or cross-contaminated. For example HeLa cells, one of the fastest growing cell lines have been shown to invade and overtake other cell lines.
Researchers working with immortalized cell lines would readily agree when I state that it is almost impossible to look at cells under the microscope and identify them by name. There are phenotypic traits, however they do change with change in media composition, passage number and in response to growth factors. I remember the pretty arborizations my neuroblastoma cell line SH-SY5Y exhibited in response to nerve growth factor treatment. Thus physical appearance is not a distinguishing feature. Currently, in many labs, researchers typically use more than one cell line, and more than likely, share the same lab space to passage cells and the same incubator to grow the cells. In such scenarios, it is not difficult to imagine that cell lines might get mislabeled or cross-contaminated. For example HeLa cells, one of the fastest growing cell lines have been shown to invade and overtake other cell lines.
Misidentification of cell lines has deep and severe implications. A review of cell lines used to study esophageal adenocarcinoma found that a large number of the cell lines were actually derived from lung or gastric cancers. Unfortunately, by the time this error was discovered, data from these cell line studies were already being used for clinical trials and other advanced studies and publications. Moreover, the cell lines were being to screen and design and test specific cancer drugs which ended up in flawed clinical trials.
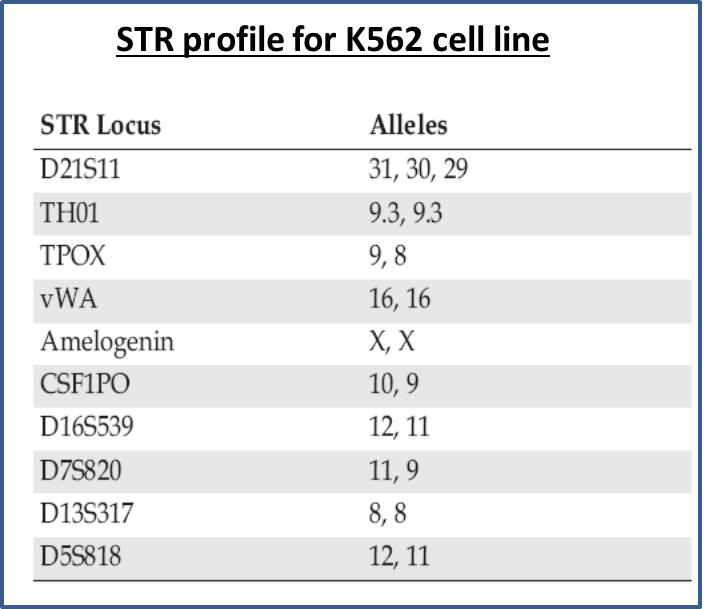 Therefore a universal and unbiased method for cell line authentication is absolutely essential. Stressing on this aspect, new guidelines were established (1) and many scientific journals require a cell line authentication report prior to accepting a manuscript for publication. Prior to DNA analysis, karyotyping and isoenzyme analysis was used for identification. Isoenzyme analysis is used to study the patterns of migration of isoenzymes present in cell lysates following electrophoresis using agarose gels. The patterns obtained are species specific and therefore are used as quality control and authentication procedures to confirm species of origin of material. However, neither karyotyping nor isozyme analysis matches the specificity and power of discrimination of DNA STR technology. Short tandem repeat (STR) sequences, as the name suggests are short, usually 3-5 basepairs in length and are repeated in tandem, and are dispersed across all across the genome. These are used as polymorphic markers and can be differentiated by the number of repeat sequences when amplified using fluorescent primers flanking the repeat sequences. Therefore, amplicons from a pentanucleotide repeat locus would differ in length by a factor of 5bp depending on the number of repeat units. A multiplex PCR generates a unique code for each human cell line analyzed (see Fig), so the DNA profile of each new stock can be verified by comparing to the baseline profile. This level of discrimination and resolution is possible only on capillary electrophoresis instruments. Also, a dedicated software is needed to analyze the STR data and generate a profile. Therefore, this is a fairly extensive and expensive setup and may not be feasible or worthwhile for labs to invest in the equipment and resources for determining the genetic profile of the cell line they are working with. However, there are many cell line authentication centers including core labs that offer this service for a fee. One of the main centers is ATCC which uses Promega’s PowerPlex® chemistry to identify and authenticate cell lines(2). The process is very simple and yet reproducibly produces reliable results. Researchers can spot cells on supplied collection card or purified frozen DNA and mail it to ATCC and receive a detailed report and STR profile of their cell line.
Therefore a universal and unbiased method for cell line authentication is absolutely essential. Stressing on this aspect, new guidelines were established (1) and many scientific journals require a cell line authentication report prior to accepting a manuscript for publication. Prior to DNA analysis, karyotyping and isoenzyme analysis was used for identification. Isoenzyme analysis is used to study the patterns of migration of isoenzymes present in cell lysates following electrophoresis using agarose gels. The patterns obtained are species specific and therefore are used as quality control and authentication procedures to confirm species of origin of material. However, neither karyotyping nor isozyme analysis matches the specificity and power of discrimination of DNA STR technology. Short tandem repeat (STR) sequences, as the name suggests are short, usually 3-5 basepairs in length and are repeated in tandem, and are dispersed across all across the genome. These are used as polymorphic markers and can be differentiated by the number of repeat sequences when amplified using fluorescent primers flanking the repeat sequences. Therefore, amplicons from a pentanucleotide repeat locus would differ in length by a factor of 5bp depending on the number of repeat units. A multiplex PCR generates a unique code for each human cell line analyzed (see Fig), so the DNA profile of each new stock can be verified by comparing to the baseline profile. This level of discrimination and resolution is possible only on capillary electrophoresis instruments. Also, a dedicated software is needed to analyze the STR data and generate a profile. Therefore, this is a fairly extensive and expensive setup and may not be feasible or worthwhile for labs to invest in the equipment and resources for determining the genetic profile of the cell line they are working with. However, there are many cell line authentication centers including core labs that offer this service for a fee. One of the main centers is ATCC which uses Promega’s PowerPlex® chemistry to identify and authenticate cell lines(2). The process is very simple and yet reproducibly produces reliable results. Researchers can spot cells on supplied collection card or purified frozen DNA and mail it to ATCC and receive a detailed report and STR profile of their cell line.
How often should one check the genetic profile? ATCC and other cell repositories authenticate all cell lines they distribute and therefore, STR profiles already exist at the beginning of the experiment. Typically it is recommended that when a new line is established a baseline STR profile must be generated using very early passages of the cell line. With new and established cell lines, profile needs to be verified during the course of experiment, especially when cell lines are expanded for freezing purposes and also at the time of manuscript submission(3). This is especially crucial if labs are using more than one cell line concurrently.
Cell lines are extremely valuable research tools and it is in the best interest of science to reduce and ultimately eliminate the use of misidentified and contaminated cell lines.
References
1. Barralon, R., et al. (2010) Recommendation of short tandem repeat profiling for authenticating human cell lines, stem cells, and tissues. In Vitro Cell. Dev. Biol. Anim. 46(9), 727-32.
2. http://www.atcc.org/en/Services/Testing_Services/Cell_Authentication_Testing_Service.aspx
3. http://www.promega.com/products/pm/cell-authentication/guidelines/
Anupama Gopalakrishnan
Latest posts by Anupama Gopalakrishnan (see all)
- Mitochondrial DNA Typing in Forensics - February 25, 2015
- PowerQuant System: Tool for informed casework sample processing decisions - July 21, 2014
- Biology of Overeating and the Weight-Gain Cycle - April 28, 2014

Reblogged this on Promega Scientific Training.