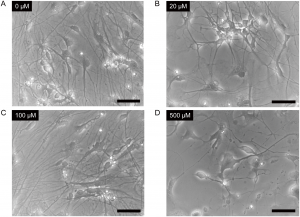
Cells were treated with 0μM (Panel A), 20μM (Panel B), 100μM (Panel C) or 500μM (Panel D) ketamine for 24 hours. Scale bar = 50μm. From Ito, H., Uchida, T. and Makita, K. (2015) Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons. PLOS ONE 10, e0128445.
doi:10.1371/journal.pone.0128445.g002
Creating an in vitro human neuronal cell model started with human induced pluripotent stem cells (iPSCs) cultured under conditions that produced neurons. After 14 days, the cells changed morphology from the initial progenitor cells and looked more like neurons. The iPSC-derived neurons were positive for βIII-tubulin and tyrosine hydroxylase staining, two markers for neuronal differentiation. Compared with a differentiated cortical neuronal cell line, the neurons differentiated from iPSCs had similar percent staining for βIII-tubulin.
Cell Morphology Effects
Next, Ito, Uchida and Makita wanted to test the system to see what kind of changes were induced in cells exposed to ketamine. When the IPC-derived neurons were exposed to ketamine in increasing concentrations (20, 100 and 500μM) for 24 hours, cells exposed to the highest dose showed distinct morphological changes. Seeing physical changes in iPSC-derived neurons exposed to 500μM ketamine, however, does not reveal what lower concentrations of ketamine might be doing at the cellular level.
Cell Health Effects
So how was cell health affected if at all? Using multiplexed cell viability and caspase-3/7 assays, the researchers looked at iPSC-derived neurons exposed to 20, 100 and 500μM ketamine for 6 and 24 hours. Regardless of the concentration of ketamine used, cells remained viable. However, for cells treated with 500μM ketamine, caspase-3/7 levels increased at both 6 and 24 hours, indicating that apoptosis was initiated. Similar to the apoptosis assay, H2O2 levels were elevated at 6 and 24 hours for iPSC-derived neurons treated with 500μM ketamine, but not for the lower concentrations. The differentiated cortical neuronal cell line showed the same increase in the levels of caspase-3/7 and reactive oxygen species (ROS) under the same conditions. To examine if there was a relationship between ROS activation and caspase-3/7 levels, iPSC-derived neurons were treated with 500µM ketamine for 6 hours with or without a ROS scavenger. For the cells treated with the ROS scavenger, both ROS levels and caspase-3/7 levels were reduced compared to cells treated with 500µM ketamine alone.
Mitochondrial Effects
Ito, Uchida and Makita next investigated whether ketamine cytotoxicity affected mitochondria. To test this, iPSC-derived neurons were treated with 20, 100 and 500μM ketamine for 6 and 24 hours, and the levels of cellular ATP assessed. Levels of ATP decreased in both a time- and dose-dependent manner for both 100 and 500µ ketamine. That is, cells treated with 500µM ketamine for 24 hours had the greatest reduction in ATP, while cells treated with 100µM ketamine for 6 hours had significant but more modest ATP reduction.
Oxidative Stress Effects
How did ketamine affect oxidative stress in neuronal cells? The researchers examined how the NADH:NAD+ ratio changed with increasing concentration of ketamine. Similar to ATP levels, the NADH:NAD+ ratios had increased when iPSC-derived neurons were treated with 100 and 500μM ketamine at both 6 and 24 hours. These same results were seen in the cortical neuronal cells. These results suggested that ketamine affected oxidative phosphorylation in mitochondria. Therefore, Ito, Uchida and Makita studied if ketamine directly affected the mitochondrial respiratory chain complex activities using an assay where NADH is oxidized to NAD+. Using ketamine-treated bovine heart mitochondrial respiratory chain complex (I, II, IV and V), researchers saw NADH dehydrogenase (complex I) and ATP synthase (complex V) significantly decreased when treated with 125 or 500μM ketamine while complex II (succinate dehydrogenase) and complex IV (cytochrome c oxidase) activities were unchanged.
Physical Effects on Mitochondria
Mitochondrial membrane potential and morphology were also examined after ketamine treatment. For both iPCS-derived neurons and cortical neuronal cells treated with 500µM ketamine for 6 or 24 hours, mitochondrial membrane potential significantly decreased compared with untreated cells. Confocal microscopy of neurons treated with ketamine showed shortened mitochondria in those cells exposed to 100µM and 500µM ketamine. Under a transmission electron microscope, neurons exposed to 100μM ketamine had fragmented mitochondria and autophagosomes present in the cytosol. Cells treated with 500µM ketamine had numerous autophagosomes in all cells scanned and had shortened mitochondria with irregular cristae.
To address neuronal functionality, iPSC-derived neurons were tested for monoamine neurotransmitter reuptake activity. Cells exposed to 100µM and 500µM ketamine had reduced reuptake activity compared to untreated cells.
Studying how medications affect humans can be difficult, and developing human model systems to study side effects is complicated. These researchers started with a known problem (ketamine cytotoxicity) and developed a potential model system to examine how ketamine was affecting neurons. Their system showed that ketamine affected both neuronal cell health and mitochondria morphology and functionality. Further study is needed to validate this model and the results of ketamine treatment.
To learn more about the cell health and metabolism assays used in this paper, visit our web site.
Reference
Ito, H., Uchida, T. and Makita, K. (2015) Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell-derived neurons. PLOS ONE 10, e0128445.
Sara Klink
Latest posts by Sara Klink (see all)
- A One-Two Punch to Knock Out HIV - September 28, 2021
- Toxicity Studies in Organoid Models: Developing an Alternative to Animal Testing - June 10, 2021
- Herd Immunity: What the Flock Are You Talking About? - May 10, 2021
