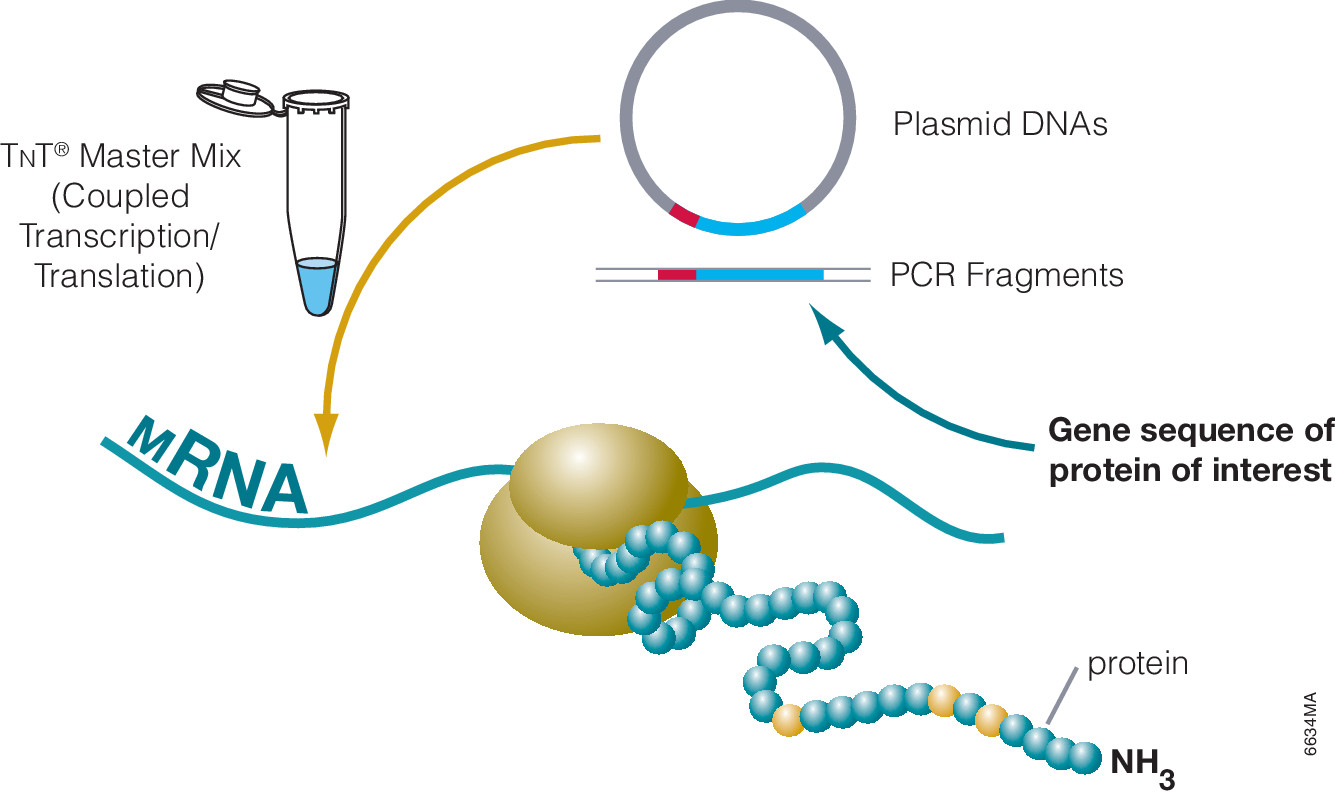
Cell-free protein expression is a simplified and accelerated avenue for the transcription and/or translation of a specific protein in a quasi cell environment. An alternative to slower, more cumbersome cell-based methods, cell-free protein expression methods are simple and fast and can overcome toxicity and solubility issues sometimes experienced in traditional E. coli expression systems.
Cell-free protein expression offers a convenient method for generating small amounts of protein for a variety of applications (e.g., protein:protein interactions, protein:nucleic acid interactions, structural analysis, functional assays and toxicity screening). This approach lends itself to specific protein labeling with fluorophores, biotin, radioactivity or heavy atoms via modified charged tRNAs or amino acids. Cell-free protein expression systems provide quick access to proteins of interest and remain a staple in the collection of tools available to elucidate protein structure and function, understanding cellular pathways and mechanisms and high-throughput screening of compounds for drug discovery. There are a number of different cell-free expressions systems, each with different strengths. Deciding which one is right for you depends upon your research needs and goals.
The S30 T7 High-Yield Protein Expression System is a prokaryotic expression system that uses an E. coli S30 extract to express proteins. The system uses T7 promoter-driven bacterial expression vectors. This system produces the highest amount of protein of all the Promega systems, up to 500µg/ml of protein. The high yields make this system ideal for high-throughput screening applications, where larger amounts of protein are needed. Because this system uses an E. coli-based extract, results with proteins expressed in the S30 T7 High-Yield Protein Expression System can be readily compared to those from proteins generated using E. coli cell-based protein expressions methods.
There are a number of different eukaryotic systems that use extracts from different sources such as plant (wheat germ), insect (Spodoptera frugiperda) and mammalian cells (rabbit reticulocyte lysates). Using different extracts gives each of these systems unique characteristics.
The plant-based TNT® SP6 High-Yield Wheat Germ System is an animal-free eukaryotic system that produces larger numbers of full-length soluble proteins than many other cell-free or cell-based systems. This system produces yields of greater than 200µg/ml of protein. The system is compatible with vectors containing either the SP6 or T7 vector. The highest yields are obtained with vectors containing plant viral 5´ and 3´ untranslated regions (UTRs).
Another choice for animal-free eukaryotic protein production is insect-based expression systems such as the TNT® T7 Insect Cell Extract System. This system produces highly active, full-length proteins with relatively high yields. In a comparison study using cAMP-dependent protein kinase A (PKA), the TNT® T7 Insect Cell Extract System produced significantly more active protein than both plant- and mammalian-based system. This system uses baculovirus expression vectors with a T7 promoter upstream of the gene sequence.
Mammalian-based TNT® Quick Coupled Transcription/Translation Systems are available for the SP6 or T7 promoters, and can use with most vectors containing either one of these promoters. This system allows mammalian proteins to be expressed in a native environment with a high likelihood of obtaining full-length proteins. The system works with a large number of T7 or SP6 expression vectors, including those intended for bacterial, insect or plant expression systems.
Cell-free protein expression offers unique advantages when compared to cell-based protein expression including time savings and increased overall yields of functional, soluble, full-length proteins. These systems are not as sensitive to toxic proteins as cell-based systsms are and are adaptable to high-throughput experiments. The wide variety of cell-free expression systems available offer researchers several options for characterizing proteins, including immunoprecipitation, pull-down experiments and enzymatic assays. When selecting a cell-free protein expression system some things to consider include the source of the extract or lysate, the template you will be using, the desired protein yield and the intended downstream application.
More information on cell-free protein expression is available in the Protein Expression Chapter of the Promega Protocols and Applications Guide.

a nice one…!
I really agree with you that this one is very important for most of the research field.