Working with RNA can be a tricky thing…it falls apart easily, and RNases (enzymes that degrade RNA) are ubiquitous. Successfully isolating RNA and maintaining its integrity is critical, especially when sensitive downstream applications are used (e.g., RNA-Seq).
Good techniques for RNA handling are simple to employ but crucial for success. All RNA purification and handling should take place in an RNase-free, RNA-only zone of the lab. Segregating RNA work from protein and DNA purification and handling will help minimize the potential for RNase contamination and help keep your RNA intact. Only buffer and water stocks treated to be RNase-free should be kept in the RNA area of the lab, and gloves should be worn at all times to prevent accidental contamination. Tools and equipment such as pipets, tips, and centrifuges should be designated for use only in the RNA zone as well. The location of the RNA zone in the lab is also important. Keeping traffic to a minimum and moving the RNA zone away from doors, windows, and vents can also help minimize contamination.
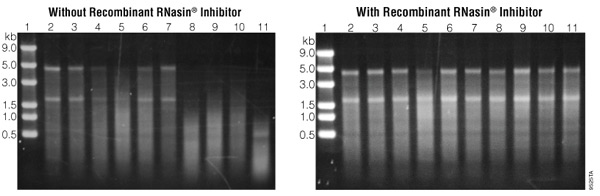
Using an RNase inhibitor can also help safeguard your samples from RNase degradation. These inhibitors can bind to any RNases that may have been introduced into your sample and prevent them from cutting the RNA present.