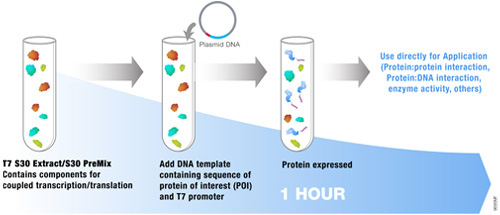
Aberrant methylation events have significant impacts in terms of incidence of cancer and development disregulation. Researchers studying DNA methylation are often working with DNA from “difficult” tissues such as formalin-fixed, paraffin embedded tissues, which characteristically yield DNA that is more fragmented than that purified from fresh tissue. Traditional methods for bisulfite conversion involve a long protocol, harsh chemicals, and generally yield highly fragmented DNA. The DNA fragmentation may significantly impact the utility of the converted DNA in downstream applications such as bisulfite-specific PCR or bisulfite sequencing.
An ideal bisulfite conversion system enables complete conversion of a DNA sample in a short period of time, provides high yield of DNA, minimally fragments the DNA, works on a wide range of input DNA amounts (from a wide variety of sample types), and, while we’re at it, is easy to use and to store. Whew! That’s quite the list.
Continue reading “A New Edge in Bisulfite Conversion”