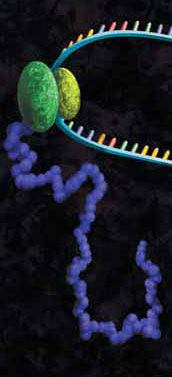
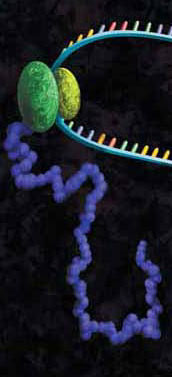
 Epigenetics is the study of the processes involved in the genetic development of an organism, especially the activation and deactivation of genes. One way that genes are regulated is through the remodeling of chromatin. Chromatin is the complex of DNA and the histone proteins with which it associates. The conformation of chromatin is profoundly influenced by the post-translational modification of the histone proteins. These modifications include acetylation, methylation, ubiquitylation, phosphorylation and sumolyation. The following references illustrate the use of cell-free expression to characterize this process.
Epigenetics is the study of the processes involved in the genetic development of an organism, especially the activation and deactivation of genes. One way that genes are regulated is through the remodeling of chromatin. Chromatin is the complex of DNA and the histone proteins with which it associates. The conformation of chromatin is profoundly influenced by the post-translational modification of the histone proteins. These modifications include acetylation, methylation, ubiquitylation, phosphorylation and sumolyation. The following references illustrate the use of cell-free expression to characterize this process.
Shao, Y. et al. (2010) Nucl. Acid. Res. 38, 2813–24.
Carbonic anhydrase IX (CAIX) plays an important role in the growth and survival of tumor cells.The MORC proteins contain a CW-type zinc finger domain and are predicted to have the function of regulating transcription, but no MORC2 target genes have been identified. CAIX mRNA to be down-regulated 8-fold when MORC2 was overexpressed. Moreover, MORC2 decreased the acetylation level of histone H3 at the CAIX promoter. Among the six HDACs tested, histone deacetylase 4 (HDAC4) had a much more prominent effect on CAIX repression. Assays showed that MORC2 and HDAC4 were assembled on the same region of the CAIX promoter. Interaction between MORC2 and HDAC 4 were confirmed by using cell free expression of MORC2 and GST-HDAC (GST pull-downs). Cell-free expression was also used to express MORC2 proteins to determine through gel shifts the binding location on the CAIX promoter region (gel shift experiments)
Denis, H. et al. (2009) Mol. Cell. Biol. 29, 4982–93.
The recent identification of enzymes that antagonize or remove histone methylation offers new opportunities to appreciate histone methylation plasticity in the regulation of epigenetic pathways. PAD4 was the first enzyme shown to antagonize histone methylation. Very little is known as to how PADI4 silences gene expression. Through the use of cell-free expression to express both PAD4 and HDAC1 proteins and E. coli expression of GST fusions of PAD4 and HDAC1, pulldown experiments confirmed by in vivo experiments that PADI4 associates with the histone deacetylase 1 (HDAC1), and the corresponding activities, associate cyclically and coordinately with the pS2 promoter during repression phases.
Brackertz, M. et al. (2006) Nucl. Acid. Res. 34, 397-406.
The Mi-2/NuRD complex is a multi-subunit protein complex with enzymatic activities involving chromatin remodeling and histone deacetylation. The function of p66α and of p66β within the multiple subunits has not been addressed. GST-fused histone tails of H2A, H2B, H3 and H4 were expressed in E. coli used in an in vitro pull-down assay with radioactively labeled p66-constructs expressed using cell free systems. Deletions at the C terminus noted reduced binding of p66 where as deletions at the N terminus did not affect binding. Also observed was that acetylation of histone tails reduces the association with both p66-proteins in vitro.
Zhou, R. et al. (2009) Nucl. Acids. Res. 37, 5183–96.
Lymphoid specific helicase (Lsh) belongs to the family of SNF2/helicases. Disruption of Lsh leads to developmental growth retardation and premature aging in mice. However, the specific effect of Lsh on human cellular senescence remains unknown. In vivo results noted that Lsh requires histone deacetylase (HDAC) activity to repress p16INK4a. Moreover, overexpression of Lsh is correlated with deacetylation of histone H3 at the p16 promoter. In vitro pull-downs using cell free expression and GST fusions from E. coli were used to collaborate interactions between Lsh, histone deacetylase 1 (HDAC1) and HDAC2 observed in vivo.
Like this:
Like Loading...
 Ubiquitination refers to the post translational modification of a protein by attachment of one or more ubiquitin monomers. The most prominent function of ubiqutin is labeling proteins for proteasome degradation. In addition to this function ubiquitination also controls the stability, function and intracellular localization of a wide variety of proteins.
Ubiquitination refers to the post translational modification of a protein by attachment of one or more ubiquitin monomers. The most prominent function of ubiqutin is labeling proteins for proteasome degradation. In addition to this function ubiquitination also controls the stability, function and intracellular localization of a wide variety of proteins. 

![0877MA10_3A-[Converted]](https://www.promegaconnections.com/wp-content/uploads/2011/06/0877ma10_3a-converted.jpg?w=300)