Scrolling through the October 6 headlines, enjoying my morning cup of coffee, I came across a piece of news that brought this chemist-turned-science writer a special sort of nerdy joy. The 2021 Nobel Prize in Chemistry had been announced, and I was going to get to write about a subject near and dear to my heart—catalysis and sustainability.
Continue reading “Catalyzing Greener Chemistry”General
The Power of Binding: Using Trivalent PROTACs to Enhance Protein Degradation
The University of Dundee in Scotland and Promega Corporation have developed a new approach to targeted protein degradation: a revolutionary “three-headed hydra” with a unique three-pronged structure that packs a powerful punch.
Five Fun Facts About Sloths and Three Things We Can Learn from Them
Sloths. These slow-moving, baby-faced, tree-dwelling mammals have risen to stardom in recent years, with their chubby, bandit-masked faces appearing on everything from socks and t-shirts to coffee mugs and post-it notes. We can all agree they are cute, but how much do we know about them?

Rare Human Antibody Could Lead to A Universal Coronavirus Vaccine
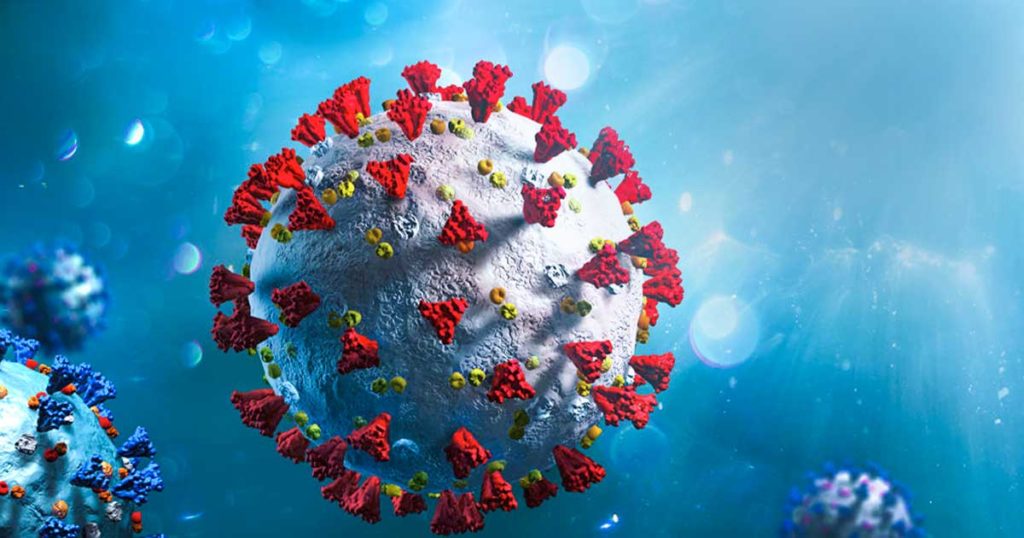
As we’ve all learned from the current global COVID-19 pandemic, coronaviruses are generally bad news. Among the four genera of coronaviruses, the betacoronavirus genus has been especially notorious. In the past 20 years, three highly pathogenic betacoronaviruses, MERS-CoV, SARS-CoV and SARS-CoV-2 have resulted in serious pandemics. All three of these viruses originated from bats, highlighting the continued risk of coronavirus transmission from animals to humans.
Typically, when a new viral threat emerges, researchers scramble to develop drugs or vaccines after the virus has already gotten out of control. However, developing a vaccine for each new virus is a slow and painstaking process, and many lives will be lost before a vaccine is distributed. But what if we had one, universal coronavirus vaccine that could neutralize not only all existing betacoronaviruses, but any new variants that emerge in the future?
Continue reading “Rare Human Antibody Could Lead to A Universal Coronavirus Vaccine”Our Future is at Hand; Global Handwashing Day
Each year, on October 15th, we celebrate Global Handwashing Day. This day aims to raise awareness about the critical importance of handwashing as well as educate and encourage people around the world to handwash with soap.

Keeping hands clean is one of the easiest, most effective, and cheapest ways we can prevent germs from spreading. As we continue to fight COVID-19, we need to leverage the lessons learned from our pandemic response and prioritize proper hand hygiene.
Continue reading “Our Future is at Hand; Global Handwashing Day”Racing Against the Clock, University of St Andrews iGEM Team Engineers Safer Sunscreen
Today’s blog was written in collaboration with Julia Ashley and AJ Ridley.
This August marked the end of a frantic 10 weeks of laboratory experiments for Julia Ashley and AJ Ridley. The two are members of the University of St Andrews iGEM team and are working to develop a product called Shinescreen, a probiotic sunscreen that is safe for marine life.
“Experiments were coming along nicely until the last two weeks in the lab. We ran into trouble with some transformation experiments,” said Ridley.
The team was on the clock, with only two and a half months of access to a laboratory. Unfortunately, the team’s time ran out before they could resolve all the issues.
“We learned the realities of science that not everything goes the way you think it will,” said Ashley. “But we got some results, and we’re happy with that.”

Fighting Extinction: Komodo Dragons At Risk
Komodo Dragons are not only the largest lizard on Earth but also one of the most ferocious species with a fearsome reputation. The carnivorous beast can grow up to 10 feet long and can detect flesh from miles away. However, the Komodo Dragon’s serrated teeth, armored scales, and venom-laced saliva are still being outmatched by its biggest competitor: extinction.
The Komodo Dragon was previously named a “vulnerable” species by the conservation organization before being reclassified as “endangered.” There is hope that this change in status will encourage policymakers and conservation groups to strengthen and expand protections.

25 years ago, there were somewhere between 5,000 and 8,000 Komodo Dragons. Today, there are an estimated 1,380 adults and 2,000 juveniles in the wild. The Komodo Dragon is moving towards extinction.
Continue reading “Fighting Extinction: Komodo Dragons At Risk”Catch Cross-Contamination Early: Authenticate Your Cell Lines!
Soon after Amanda Capes-Davis started working with CellBank Australia, she received a request from an exasperated graduate student:
This cell line was handed down to me for my project, but I’m getting strange experimental results with the cells. Can you authenticate the cell line?
After performing genetic analyses, Capes-Davis soon had the answer to the student’s experimental woes: the cells did not come from the human tissue type the student was studying. They weren’t even human—they were mouse cells.
“She’d been given this cell line that was behaving differently than expected, and people thought ‘wow, this is an exciting new variant,’ it could tell her more about a particular disease,” Capes-Davis said. “But no, it was a more sinister reason, unfortunately.”

Evidence of Inflammasome Activation in Severe COVID-19
The pandemic caused by SARS-CoV-2 has brought the world to its knees. There have been many deaths, many persons with lingering disease (long COVID) and the inability to vaccinate everyone quickly, for starters. SARS-CoV-2 has not only been a tricky adversary in terms of treatment options to save lives, it’s also been a wily opponent to researchers studying the virus.
Contributing to the existing studies, with their review of the role of inflammasomes in COVID-19, Vora et al. recently published “Inflammasome activation at the crux of severe COVID-19” in Nature Reviews Immunology. In this paper they detail evidence of inflammasome activation and its role in SARS-CoV-2 infections.
Contributions of Those Lost in the SARS-CoV-2 Pandemic
I’d like to take a moment to note the uniquely awful nature of the virus at the center of this blog and the paper it reviews. Many of the papers we blog about describe research involving cell lines, mice or another animal model. The closest most reports get to human research subjects is the use of human cells lines. In the Vora et al. report, serum and tissue samples are from actual human patients, some that survived and many that did not survive COVID-19. It’s not lost on us, Dear Reader, the contributions of those that suffered and died due to SARS-CoV-2 infection. Many persons with severe or fatal COVID-19 have made a significant contribution to our understanding of this virus and its treatment options. We owe them, as well as the researchers that have studied SARS-CoV-2, our sincerest gratitude.
Why the Interest in Inflammasomes?
For detailed information on inflammasomes you can read Ken’s blog, here. You will find background information there and on our inflammasome web page.
In their paper, Vora et al. provide evidence of inflammasome activation, both direct and indirect, in COVID-19. The authors note:
Continue reading “Evidence of Inflammasome Activation in Severe COVID-19”“Key to inflammation and innate immunity, inflammasomes are large, micrometrescale multiprotein cytosolic complexes that assemble in response to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and trigger proinflammatory cytokine release as well as pyroptosis, a proinflammatory lytic cell death.”
New Cleared IVD Assay for Microsatellite Instability in Colorectal Cancer Aims to Help Identify Those with Lynch Syndrome
Lynch syndrome is an inherited condition that significantly increases the risk of developing colorectal and other cancers, often at a young age. People with this condition have close to an 80% chance of developing colorectal cancer in their lifetime. It is the most common form of hereditary colon cancer and causes roughly 3% of all colon cancers. The mutations that cause Lynch syndrome are inherited in an autosomal dominant manner— meaning you only need to have one copy of the gene with a Lynch-associated mutation to be at an increased risk.
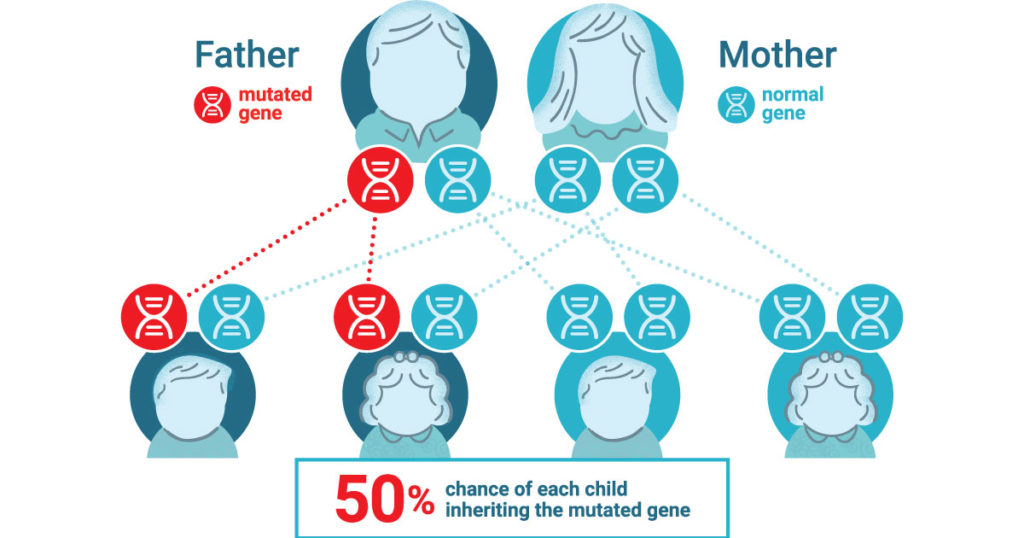
It is estimated that 1 in every 279 people have inherited a Lynch-associated mutation (1). Yet despite this prevelence, Lynch syndrome is not well known and ~95% of those with the syndrome don’t know they have it (1).
Lynch Syndrome Cause and Detection
Lynch syndrome is caused by mutations that result in the loss of function of one of the four different major mismatch repair proteins. These proteins act as “proof readers” that correct errors in the DNA sequence that can occur during DNA replication. To determine if Lynch syndrome is likely, simple screening tests can be performed on tumor (cancer) tissue to indicate if more specific genetic testing should be considered. One such screening looks for high levels of microsatellite instability (MSI) in the tumor tissue. High microsatellite instability (MSI-H) in tumor tissue is a functional indication that one or more of the major mismatch repair proteins is not functioning properly.
For those who develop colorectal cancer at an early age or have a family history (immediate family member or multiple family members with colorectal cancer or polyps), screening for Lynch syndrome can offer valuable insight for both patients and their family, as well as for their healthcare provider.
New MSI IVD Test for Colorectal Cancer to Help Identify Lynch Syndrome
The newly released Promega OncoMate® MSI Dx Analysis System is an FDA-cleared IVD Medical Device and can be used to determine the MSI status of colorectal cancer tumors to aid in identifying those who should be further tested for Lynch syndrome. The OncoMate MSI Dx Analysis System builds upon the company’s fifteen year history of supporting global cancer researchers with one of the leading standard tests for MSI status detection. The OncoMate MSI Dx Analysis System offers an improved formulation while using the same five markers that have become the gold standard for MSI detection in the research community and is referenced in over 140 peer review publications (2,3).
The OncoMate® MSI Dx Analysis System is designed to provide physicians with a functional, molecular measurement of the level of DNA mismatch repair deficiency demonstrated within their patient’s colorectal cancer tumor. MSI testing is recommended to identify candidates for further diagnostic testing for Lynch syndrome. (2–4). The System is part of a broader workflow that includes DNA extraction from FFPE tissue samples, quantitation of DNA, amplification of specific microsatellite markers using multiplex PCR, fragment separation by capillary electrophoresis, and data analysis and interpretation software. The OncoMate MSI Dx Analysis System is available in certain countries. Visit the OncoMate MSI Dx Analysis System webpage to learn more.
Promega previously announced a CE-marked version of the OncoMate MSI Dx Analysis System in France, Germany, Austria, Poland, UK, Ireland, Belgium, Netherlands, Luxembourg, Spain, Italy, Switzerland, Denmark, Sweden and Norway.
For more information about MSI solutions available from Promega visit our Microsatellite Instability Testing webpage.
References
- Win, A. K. et al. (2017) Cancer Epidemiol. Prev. 26, 404–12.
- Bacher, J. et al. (2004) Dis. Markers 20, 237–50.
- Svrek, M. et al. (2019) Bull. Cancer, 106, 119–28.
- Umar, A. et al. (2004) J. Natl. Cancer Inst. 18, 261–8.
