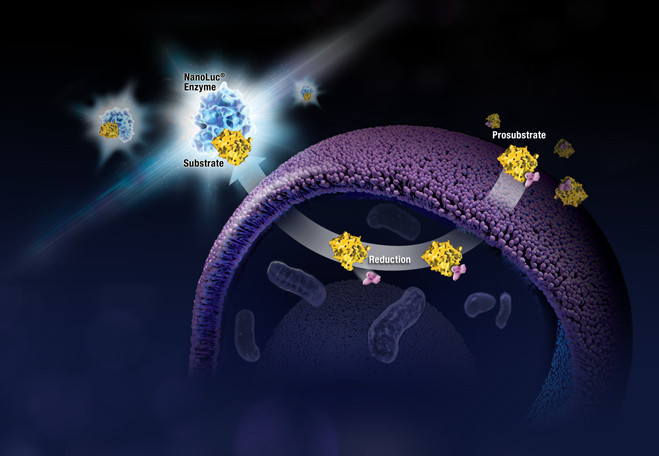
A new study, published in the Journal of Molecular Diagnostics (1), highlights the potential of using long mononucleotide repeat (LMR) markers for characterizing microsatellite instability (MSI) in several tumor types. The paper is a result of a collaborative effort between researchers from Johns Hopkins University and Promega to evaluate the performance of a panel of novel LMR markers for determining MSI status of colorectal, endometrial and prostate tumor samples.
Microsatellite instability (MSI) is the accumulation of insertion or deletion errors at microsatellites, which are short tandem repeats of DNA sequences found throughout the genome. MSI in cancerous cells is the result of a functional deficiency within one or more major DNA mismatch repair proteins (dMMR). PCR-based MSI testing is a commonly used method that can help understand a tumor’s genomic profile as it relates to MMR protein function.
Historically, MSI has been a biomarker associated with Lynch syndrome, the hereditary predisposition to colorectal and certain other cancers. In recent years, research interest in MSI has exploded, driven by the discovery that its presence in tumor tissue can be predictive of a positive response to anti-PD-1 immunotherapies (2,3).
Continue reading “New Study Suggests Long Mononucleotide Repeat Markers Offer Advantages for Detecting Microsatellite Instability in Multiple Cancers”