As we continue navigating the challenges presented by COVID-19, several research areas are crucial for helping us slow the infection rate and ending the pandemic. Advanced testing methods, such as antibody testing, help us understand and predict how the virus will spread, which can inform policy decisions. Effective therapeutics will influence clinical outcomes for individual patients, and several drugs are already being tested or administered. However, an effective vaccine against the SARS-CoV-2 virus is perhaps the most important tool we can use to protect individuals and populations from COVID-19.
Over 90 vaccines against the SARS-CoV-2 virus are currently in development around the world. While there are many different types of vaccines, the overall goal is to create long-lasting protective immunity by stimulating the production of specific antibodies. As these vaccine candidates are further characterized, monitoring ADCC activity can provide important insights into their potential efficacy.
Antibody-Dependent Cellular Cytotoxicity (ADCC) in Vaccine Development
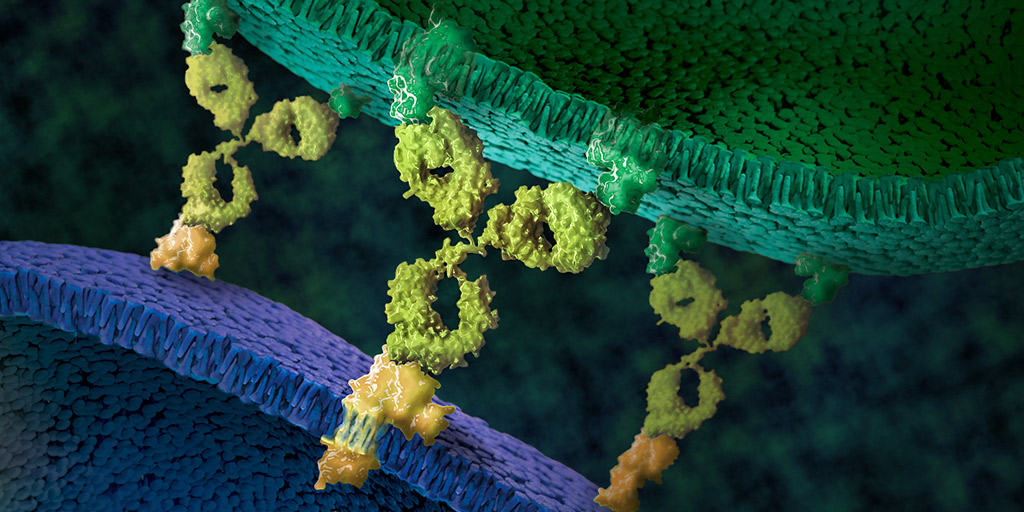
Many antibodies resulting from vaccination or infection provide immunity by neutralizing the virus particles. When the antibody binds to the surface proteins of the virus, they prevent it from entering host cells. However, researchers working on developing a universal influenza vaccine have recently given more attention to the function of key immune receptors fond on the surface of cells. A key example is the Fc class of receptors that serve as a link between effector cells and the antibody-mediated immune response. These receptors are integral to the immune mechanism known as antibody-dependent cellular cytotoxicity (ADCC).
During ADCC, the immune system lyses cells that have been infected with a particular pathogen. The process involves the activation of natural killer (NK) cells by antibodies bound to antigens on an infected cell, thus creating a bridge between innate and adaptive immunity. When immunoglobulin G (IgG) binds to a viral antigen, the Fc region of the antibody crosslinks with the Fc gamma receptor IIIα (FcγRIIIa) on NK cells. This activates a Ca2+-dependent signaling pathway that eventually causes the release of cytotoxic granules, inducing apoptosis of the target cell. In this way, antibodies that mediate ADCC are able to eliminate infected cells, instead of only neutralizing the virus particles.
As Jegaskanda writes in Vaccines (2018), labeling antibodies as “neutralizing” or “ADCC-mediating” ignores the ability of many antibodies to make use of multiple effector functions. For example, many influenza vaccines produce antibodies that bind to either the head or stem of the viral hemagglutinin (HA) protein. Some of the stem-binding antibodies bind a broader range of HA proteins, rather than specific strains. These antibodies are particularly promising for universal influenza vaccine research. However, some of these antibodies that provide broad immunity in vivo show low levels of neutralization activity in vitro. One reason is that many classical in vitro assays used for antibody characterization fail to monitor Fc effector immune functions. Assays that investigate these mechanisms are important to provide a more comprehensive picture of immune function.
As scientists around the world push forward on SARS-CoV-2 vaccine development, it will be important to monitor functions such as ADCC in order to identify the most effective candidates to confer immunity.
Learn more about our products to support Viral Research, Vaccine and Therapeutic Development.
Monitoring ADCC Activity Using Reporter Bioassays
ADCC activity can be measured in multiple ways. There are methods for measuring the cytotoxicity of target cells, including lactate dehydrogenase detection. Flow cytometry can also be used to measure target cell killing with fluorescence. The ADCC Reporter Bioassay, available from Promega, overcomes many of the limitations of other methods to provide an easy, consistent assay.
The ADCC Reporter Bioassay eliminates the need for primary cell culture by substituting a stable Jurkat cell line expressing human FcγRIIIa. When an antibody bound to antigens on the target cells binds to the FcγR receptor, the NFAT pathway is activated to induce expression of luciferase. This assay can easily be scaled to high throughput methods and shows less variability than those using primary cells.
The ADCC Reporter Bioassay has been used in several publications focusing on universal influenza vaccine development (examples provided below) and there is preprint data available on bioRxiv demonstrating its use with COVID-19 vaccine candidates.
References
- Jegaskanda S. (2018) The Potential Role of Fc-Receptor Functions in the Development of a Universal Influenza Vaccine. Vaccines 6.
- Vanderven HA, et al. (2017) Antibody-dependent cellular cytotoxicity and influenza virus. Current Opinion in Virology 22:89-96.
- Wang S-C. et al. (2019) Development of a universal influenza vaccine using hemagglutinin stem protein produced from Pichia pastoris. Virology 526:125-137.
Explore the many tools and resources we have for virus research and vaccine development on our website.
Related Posts
Latest posts by Jordan Villanueva (see all)
- Tackling Undrugged Proteins with the Promega Academic Access Program - March 4, 2025
- Academic Access to Cutting-Edge Tools Fuels Macular Degeneration Discovery - December 3, 2024
- Novel Promega Enzyme Tackles Biggest Challenge in DNA Forensics - November 7, 2024
