Proto-oncogenes are genes that organisms rely on for normal growth and development but, when mutated or dysregulated, can cause cells to grow uncontrollably, resulting in cancer and metastasis. In some cases, a single DNA mutation is sufficient for cancer to develop. Why then, do so many proto-oncogene promoters contain strings of guanine residues, which are extremely vulnerable to DNA damage from factors such as oxidative stress and hyperinflammation, to control transcription levels? From an evolutionary viewpoint, this is a contradiction: DNA sequences that are the most vulnerable to damage and mutation are key to regulating one of the cell’s most dangerous classes of genes. This seems to be a recipe for genomic instability and disease. Fortunately, evolution has provided a very clever solution to this potential problem.
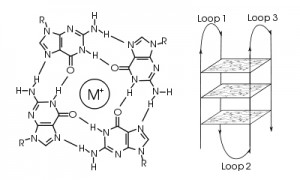
In the context of a gene promoter, these G-rich regions serve an important regulatory purpose. Four G residues associate through Hoogsteen base pairing to form a square planar structure named a G tetrad. Three G tetrads stack on top of each other to form a G-quadruplex, which is stabilized by a cation such as potassium. DNA residues not involved in the G-tetrad form loop structures. These G-quadruplexes are implicated in both upregulation and downregulation of gene expression. Scientists are discovering an increasing number of proto-oncogenes that contain these complex secondary structures within their promoter regions such as vegf, c-myc, bcl-2, hif-1α and ras.
Scientists know that these G-rich regions are hotspots for genomic instability. However, in vitro studies have shown that these G-quadruplexes are not substrates for OGG1, NEIL1, NEIL2, NEIL3 or NTH1 DNA glycosylases, which catalyze base excision repair (1). A recent ACS Central Science article has provided an explanation of how cells repair damage to these G-quadruplexes (2): Cells rely on a fifth track of G residues to temporarily replace the defective G-track, allowing DNA repair enzymes to recognize and repair DNA damage. The authors of this paper refer to this fifth G-track as a “spare tire”.
In this article, the authors monitored formation of the G-quadruplex secondary structure in two variations of the vascular endothelial growth factor (VEGF) promoter: one that contains four G-tracks and the wildtype sequence, which contains five G-tracks. For each variant, they designed oligomers that contained oxidation products of guanine at specific positions to try to disrupt formation of one of the three G-tetrads. They then examined the secondary structures of the oligomers by comparing thermal melt (Tm) profiles, the number of bound potassium ions, circular dichroism spectra and susceptibility of G residues to alkylation by dimethyl sulfate, which is blocked by Hoogsteen base pairing. The authors confirmed that incorporating oxidation products at core positions in the G-quadruplex, but not loop positions, disrupted G-tetrad formation, and thus G-quadruplex formation, in the variant with only four G-tracks. For the variant with five G-tracks, they determined that when one of the first four G-tracks of the vegf promoter was damaged, the fifth G-track was recruited to replace the defective G-track and preserve G-quadruplex structure. Meanwhile, the damaged G-track looped out and became accessible to DNA glycosylases that catalyzed base excision repair and reversed the DNA damage; this repair did not occur in the absence of the fifth G-track. Thus, this fifth G-track participates in G-quadruplex formation and presumably maintains proper gene regulation even when one of the primary G-tracks suffered oxidative damage.
I think we can all agree that this is simple and elegant solution to the original question “How do cells prevent mutations in these damage-prone G-rich regions from interfering with proper proto-oncogene expression”. During the course of evolution, these regulatory regions have acquired a “spare” G-track. Just like the spare tire of a car, this fifth G-track is at the ready to replace a damaged G-tetrad, stepping in temporarily until the main G-track can be repaired and resume its regulatory function.
- Zhou, J. et al. (2015) The NEIL glycosylases remove oxidized guanine lesions from telomeric and promoter quadruplex DNA structures. Nucleic Acids Res. 43, 4039−54.
- Fleming, A.M. et al. (2015) A role for the fifth G‑track in G‑quadruplex forming oncogene promoter sequences during oxidative stress: Do these “spare tires” have an evolved function? ACS Central Science. DOI: 10.1021/acscentsci.5b00202
