Today’s blog is written by guest blogger, Kai Hillman, Associate Product Marketing Manager at Promega.

RNA therapeutics have revolutionized modern medicine, offering groundbreaking solutions for diseases that were once deemed untreatable. These innovative treatments harness the power of RNA molecules to correct genetic anomalies and modulate protein expression, paving the way for personalized medicine. Among the many facets of RNA biology, double-stranded RNA (dsRNA) plays a pivotal role in cellular processes and immune surveillance.
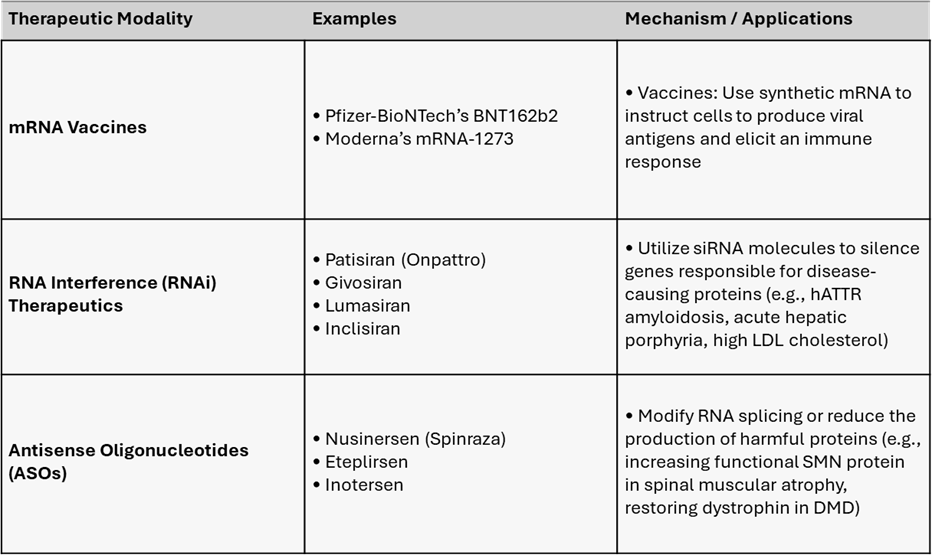
I’ve heard of mRNA, but what is ‘double-stranded RNA’?
Double-stranded RNA (dsRNA) naturally forms within cells as a byproduct of normal cellular processes or viral infections, signaling potential threats to the immune system. In RNA therapeutics, unintended formation of dsRNA can activate immune responses, potentially reducing treatment efficacy and affecting patient safety
In healthy cells, dsRNA acts as a crucial alarm signal that activates innate immune defenses through pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and RIG-I-like receptors, initiating antiviral responses1. Although essential for protecting the host against pathogens, this immune activation presents significant challenges in developing safe and effective RNA-based therapies2.
During the development and manufacturing of RNA-based treatments, unintended dsRNA structures can activate these immune pathways. The resulting immune activation may lead to inflammation and even severe adverse effects, thus compromising the therapeutic safety and efficacy. Early detection of dsRNA is crucial in preclinical research to identify and mitigate these risks. Therefore, implementing robust dsRNA detection methods is critical for ensuring safety and for refining therapeutic designs that minimize immune-related complications.
Advancements and Techniques in dsRNA Detection
In response to the challenges posed by dsRNA in RNA therapeutics, a range of advanced detection technologies has emerged. Traditional immunoassays, like ELISAs, leverage antibodies that bind to double-stranded RNA structures. Complementing these methods, high-throughput sequencing techniques allow researchers to map dsRNA presence with high resolution, while advanced imaging methods enable the visualization of dsRNA within cellular contexts.
Despite these advancements, each technology comes with its own set of limitations regarding sensitivity, specificity, and scalability—factors that are crucial for industrial application. Innovations in next-generation biosensors promise to overcome many of these hurdles, offering more precise and reliable dsRNA detection. These emerging methods are already integrating into discussions, such as the USP Virtual mRNA Therapeutics Summit, surrounding quality control protocols within therapeutics development pipelines. By combining data from bioinformatics, molecular biology, and clinical research, multidisciplinary teams are working to refine these detection techniques.
Conclusion
In summary, the detection of dsRNA is pivotal to the advancement of RNA therapeutics. It is crucial for safeguarding patients against unintended immune responses that could compromise treatment efficacy and safety. As RNA-based treatments continue to evolve, robust dsRNA detection methods, like the Lumit® dsRNA Detection Assay and TLR3 Bioassay, will play an increasingly important role in preclinical testing and quality control. The integration of innovative detection technologies and multidisciplinary collaboration promises not only to mitigate current risks but also to unlock the full potential of RNA therapeutics. Researchers and industry stakeholders are encouraged to prioritize dsRNA surveillance, ensuring that future therapies are both groundbreaking and safe, enhancing patient outcomes and driving the next generation of medical innovations.
Continuing the Conversation on dsRNA in RNA Therapeutics
This blog is the first in a three-part series exploring the role of double-stranded RNA in mRNA therapeutics. Up next, we take a closer look at common quality control pitfalls and strategies for improving dsRNA detection in IVT, followed by a discussion of how modified nucleotides impact dsRNA dynamics. Stay tuned to Promega Connections for more!
- J. Nelson, E. W. Sorensen, S. Mintri, A. E. Rabideau, W. Zheng, G. Besin, N. Khatwani,
S. V. Su, E. J. Miracco, W. J. Issa, S. Hoge, M. G. Stanton, J. L. Joyal, Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 6, eaaz6893 (2020). ↩︎ - Zhang C, Maruggi G, Shan H and Li J (2019) Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 10:594. doi: 10.3389/fimmu.2019.00594 ↩︎
Latest posts by Promega (see all)
- Immune Surveillance Meets Innovation: The Critical Need for dsRNA Detection - April 22, 2025
- Beyond Ozempic: The New Frontier of Obesity Research - April 18, 2025
- One Health and H5N1: Promega’s Commitment to Holistic Solutions - April 8, 2025
