Some of our most advanced medicines today rely on components derived from living organisms. These therapeutics, called biologics, include things like vaccines, blood products like Human Blood Clotting Factor VIII (FVIII), antibodies and stem cells. Biologics are incredibly temperature sensitive, which means they need to be kept cold during production, transport and storage, a process collectively called the cold chain. The stringent transport and storage temperature requirements for biologics create a barrier to accessing these lifesaving options; particularly for those in remote or underdeveloped regions, where maintaining a cold chain is logistically difficult and costly.
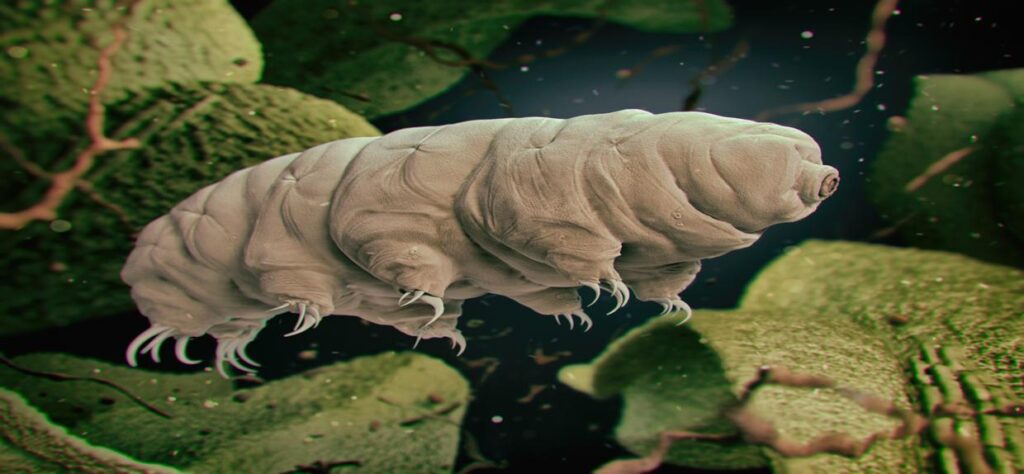
But what if we could break the cold chain? Inspired by one of the most resilient creatures on Earth – the tardigrade – scientists at the University of Wyoming are exploring ways to do just that.
Tardigrades: Nature’s Ultimate Survivors
Tardigrades (a.k.a., water bears or moss piglets) are ubiquitous microscopic invertebrates known for their ability to survive even the most extreme environmental conditions. They can endure dehydration, freezing, radiation, and even the vacuum of space by entering a dormant state of cryptobiosis, referred to as the tun state. In this state, their metabolism slows down, they reduce their need for oxygen, and they rid their cells almost completely of water. They can exist in this suspended state for extended periods, and once conditions improve, they quickly return to normal—all they need is water. This tun state has helped tardigrades survive all five mass extinction events on Earth (1).
Tolerating Desiccation by Stabilizing Proteins with Sugars and Gels
So, how do tardigrades do it? The secret lies in desiccation tolerance, a natural mechanism that stabilizes their cells and prevents damage during drying. To protect themselves, tardigrades use a mix of proteins and sugars. Although the exact pathways behind tardigrades’ incredible resilience are not known, some molecules involved have been identified. The sugar, trehalose moves into their cells to replace lost water, forming glass-like structures (vitrification) that prevent their cells from collapsing (2). Proteins called Cytoplasmic Abundant Heat Soluble (CAHS) proteins are expressed at high levels during tun formation and polymerize to form gels that slow diffusion, thus protecting desiccation-sensitive proteins from becoming nonfunctional (3,4).
Using these natural protectants as inspiration, scientists at the University of Wyoming tried applying them to biologic therapeutics with the goal of finding a way that these temperature-sensitive compounds could be stored without refrigeration.
Stabilizing Human Blood Clotting Factor VIII
The biologic FVIII is a critical blood-clotting protein used to treat hemophilia A, a genetic disorder that affects blood clotting. Like other biologics, FVIII is temperature sensitive and typically requires cold storage. Building on their previous work with trehalose and CAHS proteins from tardigrades, the scientist explored adapting them to stabilize FVIII (3,4).
They found that both sugar-based protectants (like trehalose) and protein-based protectants (like CAHS D proteins) could prevent the degradation of FVIII under repeated cycles of drying and rehydration. However, the CAHS proteins offered a unique advantage in that they could be engineered to optimize protection, making them more flexible and reliable under a range of conditions.
For example, by tweaking the structure of CAHS D, the researchers created variants that either promoted or inhibited the formation of protective gels. The gels effectively insulated FVIII from damage during thermal stress, allowing it to remain stable even when exposed to extreme heat (3).
The researchers created two main variants of CAHS D proteins, a 2X Linker Variant and a linker region (LR) variant. The 2X Linker Variant formed gels that created a stable matrix around FVIII at low concentrations. The matrix slows molecular motion and protects the protein from denaturation, effectively protecting FVIII from heat stress.
The Linker Region (LR) Variant was engineered so that it didn’t form a gel whose rigid structure could interfere with the drying process. This made the LF variant more effective at stabilizing FVIII across a wide range of conditions, particularly during repeated cycles of drying and rehydration. Using the LR variant, they were able to extend the shelf life of dry FVIII to over 10 weeks.
These results suggest that engineered proteins such as 2X Linker and LR variants offer a unique advantage over sugar-based methods because they can be customized to specific stress conditions (i.e., extreme heat or dehydration) providing a more versatile solution for biologic storage.
Looking Beyond Breaking the Cold Chain
Breaking the cold chain for biologics could have a huge impact on global healthcare, making lifesaving therapeutics easily accessible to healthcare providers in areas where the cold chain infrastructure is fragile or incomplete. The results of this study suggest that creating biologic therapeutics that are free from the cold chain is possible.
Although this research focused on using tardigrade-inspired proteins to stabilize biologics, the potential applications of these proteins could go far beyond healthcare. Imagine being able to store food or other perishable items at room temperature for extended periods. The insights gained from this research could lead to innovations in fields ranging from space exploration to agriculture and disaster relief. And all from the microscopic, resilient waterbear.
References
- Mapalo, M.A., Wolfe, J.M. & Ortega-Hernández, J. (2024) Cretaceous amber inclusions illuminate the evolutionary origin of tardigrades. Commun Biol 7, 953.
- Nguyen, K., KC, S., Gonzalez, T. et al. (2022) Trehalose and tardigrade CAHS proteins work synergistically to promote desiccation tolerance. Commun Biol 5, 1046.
- Sanchez-Martinez, S., Ramirez, J.F., Meese, E.K. et al. (2023) The tardigrade protein CAHS D interacts with, but does not retain, water in hydrated and desiccated systems. Sci Rep 13, 10449.
- Packebush, M.H., Sanchez-Martinez, S., Biswas, S. et al. (2023) Natural and engineered mediators of desiccation tolerance stabilize Human Blood Clotting Factor VIII in a dry state. Sci Rep 13, 4542.
Explore our portfolio of functional bioassays, immunoassays and protein characterization tools to accelerate your biologics drug discovery and development.
