
Antimicrobial resistance (AMR) threatens the effective prevention and treatment of an ever-increasing range of infections. It’s a leading mortality factor worldwide, but the newly discovered antibiotic, clovibactin, may offer a pivotal solution. It effectively kills drug-resistant bacterial pathogens without detectable resistance—even multidrug-resistant “superbugs.”
Only a small number of new antibiotics have been introduced in the last decade and they have often resembled their predecessors. Clovibactin stands out from other antibiotics because it was isolated from unculturable bacteria that had never encountered this antibiotic before, meaning it had no time to develop resistance.
Breaking Down the Breakthrough
Scientists at NovoBiotic Pharmaceuticals partnered with microbiologist Kim Lewis, PhD, professor at Northeastern University to develop a device called iCHip, a method of culturing bacteria that facilitated the discovery of clovibactin in a bacterium isolated from a sandy soil from North Carolina. The researchers conducted a study that effectively treated mice infected with Staphylococcus aureus, proving that clovibactin can successfully attack a broad spectrum of bacterial pathogens. They dissected clovibactin by using biochemical assays, solid-state nuclear magnetic resonance, and atomic force to find that it has an unusual killing mechanism that targets three different precursor molecules (C55PP, lipid II, and lipid IIIWTA), all of which are essential for the construction of the cell wall.
Clovibactin uses an atypical hydrophobic interface to tightly wrap around pyrophosphate while avoiding interaction with the variable structural components of precursor molecules, accounting for its resistance-lacking characteristic. It’s like a cage that encloses its target, an idea reflected in the name “clovibactin” which was derived from the Greek word “Klouvi,” meaning cage.
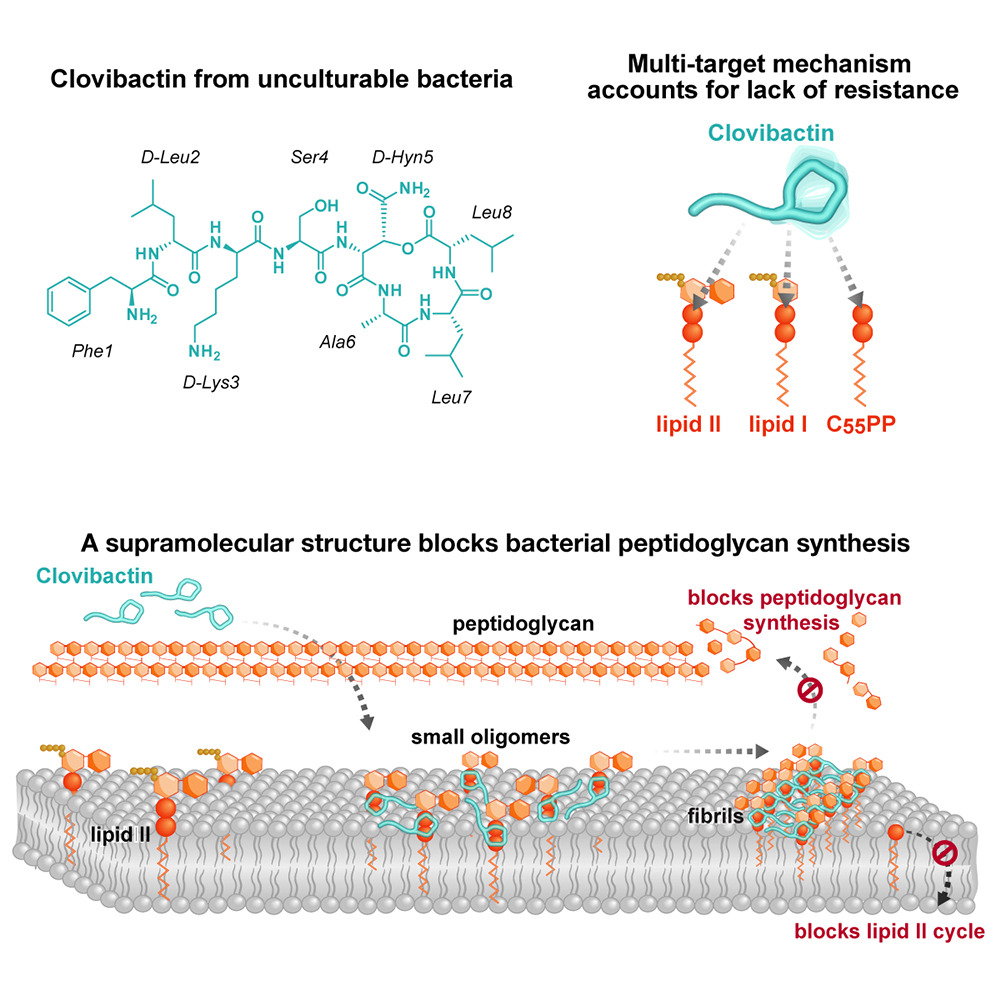
Once clovibactin binds to the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. The fibrils stay stable for a long time, ensuring that the target molecules remain sequestered until the bacteria is eliminated.
These fibrils only form on bacterial membranes that contain lipid-anchored pyrophosphate groups, not human membranes. Therefore, clovibactin selectively damages bacterial cells but is not toxic to human cells. This poses the potential for clovibactin to design improved therapeutics in the future that kill bacterial pathogens without the development of AMR.
Promega’s CellTiter 96® AQueous One Solution Cell Proliferation Assay was used to examine cytotoxicity in this study. To learn more about our assays, please visit our website.
Related Posts
Riley Bell
Latest posts by Riley Bell (see all)
- Genetic Symphonies: Building Hox of Life - December 17, 2024
- Exploring the World Through the Promega and Eppendorf Family Exchange Program - November 19, 2024
- Lab Safety: The Catalyst for Every Great Experiment - September 26, 2024

Great Revolution on Bacterial infection Treatment !