Engineered T-cell therapies, specifically CAR-T cell therapies, have emerged as a breakthrough treatment for certain types of blood cancers including lymphomas, some forms of leukemia, and most recently, multiple myeloma. CAR (chimeric antigen receptor) T-cell therapy involves collecting T cells from a patient and re-engineering them to detect and destroy cancer cells.
How are CAR-T Cells Generated?
The process of generating CAR-T cells takes place over a few weeks and can be broken down into five important steps:
- Collection: T cells are obtained from the patient’s blood plasma through a process called leukapheresis.
- Genetic modification: The collected cells are genetically modified and activated to accept a viral vector containing the genetic construct for the CAR. The CAR construct is inserted into the genome of the T cells.
- Expansion: The modified T cells are cultured and expanded in the laboratory to generate a larger population of CAR-T cells.
- Infusion: The expanded CAR-T cells are infused back into the patient’s bloodstream.
- Activation: The CAR-T cells locate and recognize the cancer cells. They become activated by binding to the cells, initiating a strong immune response against the cancer.
Quality Control: STR Analysis
The personalized nature of patients providing their unique T cells for CAR-T cell therapy presents a few quality control challenges. The safety, purity, and potency of the manufacturing process is crucial to ensure a quality end product.
One important tool that’s used to address challenges with establishing fidelity of cell identity is short tandem repeat (STR) analysis. STR analysis is a genetic identification technique used to establish paternity and identity. This informative approach to genetic identification is widely employed in the forensics community and is deemed the recommended method for authenticating research cell lines.
In the context of CAR-T cell therapy, STR analysis helps verify the identity and track the lineage of T cells and CAR-T cells from the initial sample collection all the way to the final product. The ability to analyze specific regions of the DNA, known as short tandem repeats, at all stages of the process allows scientists to compare STR profiles of the collected T cells, genetically modified CAR-T cells, and the patient’s own cells to ensure the resulting profile matches the original. Confirming cell identity and having the ability to track these cells at every stage of the manufacturing process prevents unforeseen mix-ups or sample contamination.
STR Analysis for Your Lab
Interested in implementing STR analysis for your lab? Fortunately for you, our benchtop sequencer, the Spectrum Compact CE System1, has you covered! It’s an easy-to-use, efficient interface with a complete DNA isolation, amplification, and DNA fragment analysis workflow.
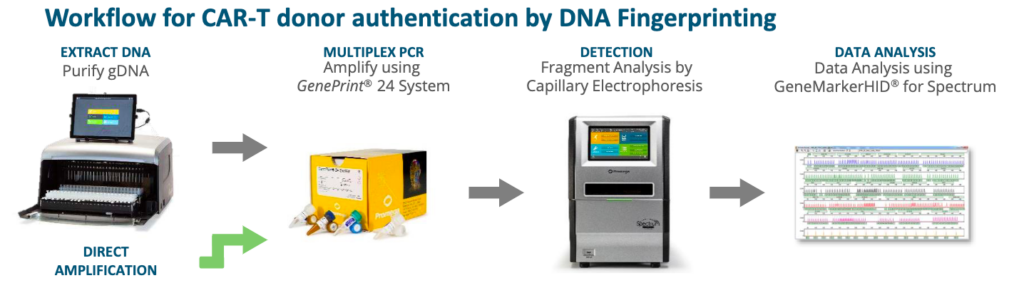
Learn more about the instrument and chat with a scientist about how the Spectrum Compact CE System fits into your workflow on our website.
- For Research Use Only. Not for use in diagnostic procedures.
The GenePrint® 24 System combined with the Spectrum Compact CE System provides highly informative STR analysis for cells used in CAR-T Cell Manufacturing. Learn more here.
