On November 18, 2022, the US Food and Drug Administration (FDA) announced the approval of the first drug to delay the onset of stage 3 type 1 diabetes (T1D). The monoclonal antibody (mAb) drug, teplizumab, was approved for use in adults and pediatric patients 8 years and older.
The road to approval has been a bumpy one for the manufacturer, Provention Bio. In 2020, the FDA rejected the application for teplizumab due to several concerns, including the small size of the clinical trial. With the current approval, based on new clinical trial results, Provention Bio confirmed a co-promotion agreement with Sanofi US. The agreement included a $35 million Sanofi equity investment in the company.
What Is Type 1 Diabetes?
Type 1 diabetes (T1D) is a chronic condition that affects millions of people worldwide. Unlike the more common type 2 diabetes (T2D), T1D is characterized by early onset and is sometimes referred to as juvenile-onset diabetes. The fundamental difference between T1D and T2D is that T1D is an autoimmune disease in which the pancreas produces little or no insulin. Therefore, people with T1D need an external supply of insulin, either by injections or with an insulin pump, in order to survive. In T2D, the pancreas can still make some insulin, but cells develop resistance to insulin and eventually stop responding to its effects. As the disease progresses, T2D patients may also require insulin supplementation. If left untreated, both types of diabetes lead to uncontrolled blood glucose levels, which can cause significant damage to the circulatory and nervous systems.
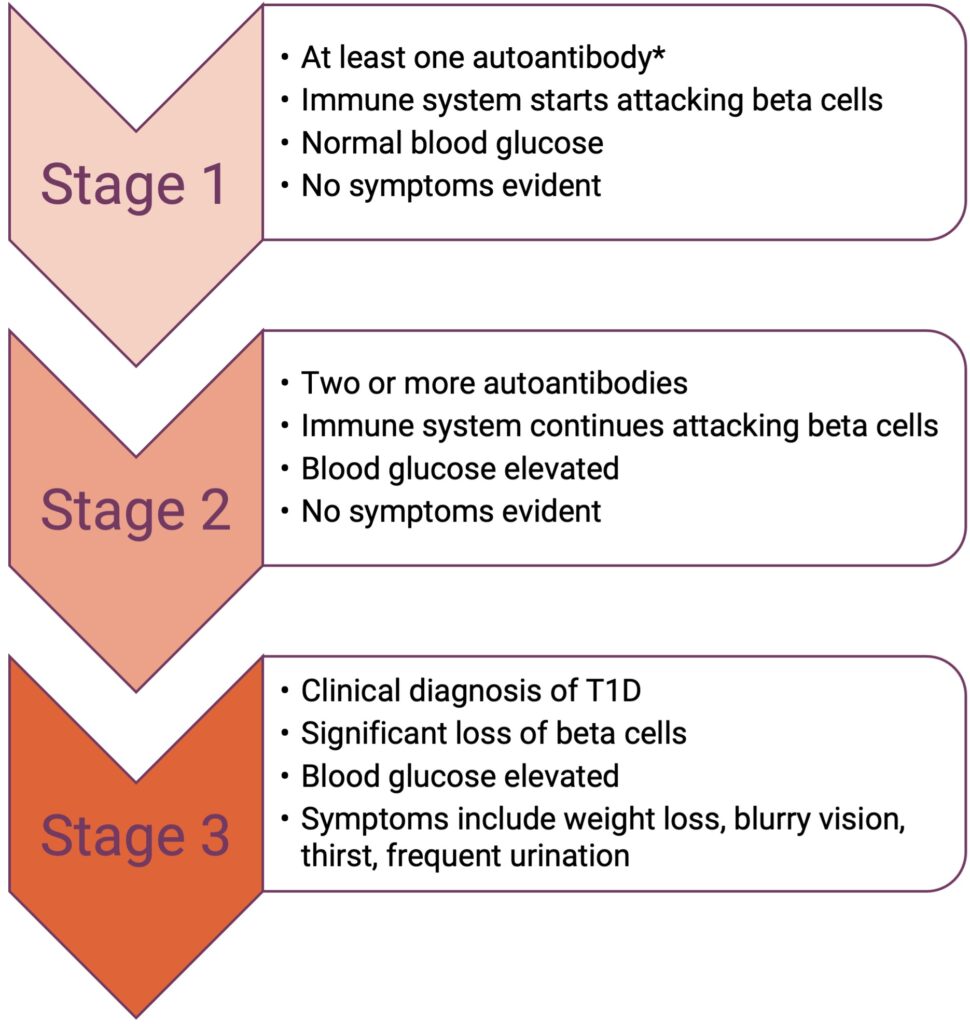
In T1D, the immune system attacks and destroys pancreatic insulin-producing beta cells, resulting in reduced insulin secretion. The disease is characterized by three stages.
How Does Teplizumab Work?
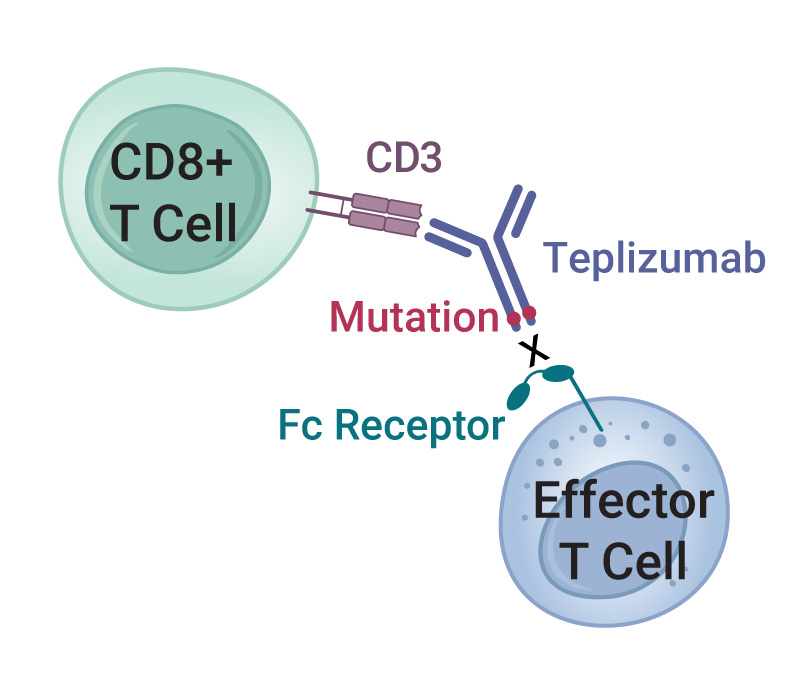
Teplizumab is a humanized mAb that recognizes the CD3 cell-surface protein complex on T cells. CD3 is involved in activating both helper and cytotoxic T cells as part of the immune response. An engineered mutation in the Fc region of teplizumab greatly reduces its binding to the Fc receptor, thereby reducing adverse effects from cytokine release syndrome (1). Although the exact mechanism of action is not clearly understood, teplizumab reduces the autoimmune destruction of pancreatic beta cells by multiple mechanisms that include blocking some types of T cells and reducing expression of genes associated with T cell activation (2).
Earlier clinical trials of teplizumab showed mixed results. A large, multicenter phase 3 trial did not show any significant differences in primary and secondary outcomes between placebo and treatment groups after 1 year (3). However, after 2 years, teplizumab reduced C-peptide loss (a measure of endogenous insulin levels) at doses that were well tolerated by patients (4). A small study in 58 patients with T1D demonstrated that the mAb reduced insulin dosage requirements in some patients (5). A more recent clinical trial in 76 patients showed that patients receiving teplizumab had a reduced risk of developing T1D compared to the placebo group (15% vs. 36%, respectively) (6).
The current study that led to FDA approval of teplizumab in stage 2 T1D patients initially showed that the drug could delay the onset of T1D by over 2 years in some patients. Follow-up results further supported the benefits of teplizumab in delaying T1D in high-risk patients (7). Additional clinical trials are ongoing and should provide further insight into whether the delay in T1D onset can be extended for a longer period. This therapeutic approach could offer new hope to patients with T1D and have a significant impact following early diagnosis.
Learn about functional bioassays for mAb drug discovery with our Biologics Resources.
References
- Gaglia, J. and Kissler, S. (2019) Anti-CD3 antibody for the prevention of type 1 diabetes – a story of perseverance. Biochemistry 58(40) 4107–4111.
- Tooley, J.E. et al. (2016) Changes in T cell subsets identify responders to FcR non-binding anti-CD3 mAb (teplizumab) in patients with Type 1 diabetes. Eur. J. Immunol. 46(1), 230–241.
- Sherry, N. et al. (2011) Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 378(9790), 487–497.
- Hagopian, W. et al. (2013) Two-year results from the randomized, placebo-controlled Protégé Trial. Diabetes 62, 3901–3908.
- Herold, K.C. et al. (2013) Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62, 3766–3774.
- Herold, K.C. et al. (2019) An Anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381(7), 603–613.
- Sims, E.K. et al. (2021) Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci. Transl. Med. 13, eabc8980.
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023
