COVID-19 cases are now being identified primarily among unvaccinated individuals, according to data from the US Centers for Disease Control and Prevention (CDC). However, there has been increasing concern about so-called breakthrough infections among fully vaccinated individuals, particularly after the emergence of the SARS-CoV-2 Delta variant.

What is a breakthrough infection? The CDC defines it as “the infection of a fully vaccinated person.” The key finding remains that people with breakthrough infections are still far less likely to experience severe COVID-19 symptoms, in contrast with unvaccinated people; hence the importance of vaccination.
Systemic and Mucosal Immunity
Why do breakthrough infections occur? COVID-19 is a disease affecting the lower respiratory tract (the trachea, primary bronchi and lungs). Currently approved vaccines, delivered via intramuscular injection, are effective at generating a systemic immune response (mediated primarily by immunoglobulin G or IgG) that neutralizes the virus in the lungs. However, the point of entry for SARS-CoV-2 is the upper respiratory tract (the nasal cavity, mouth and throat).
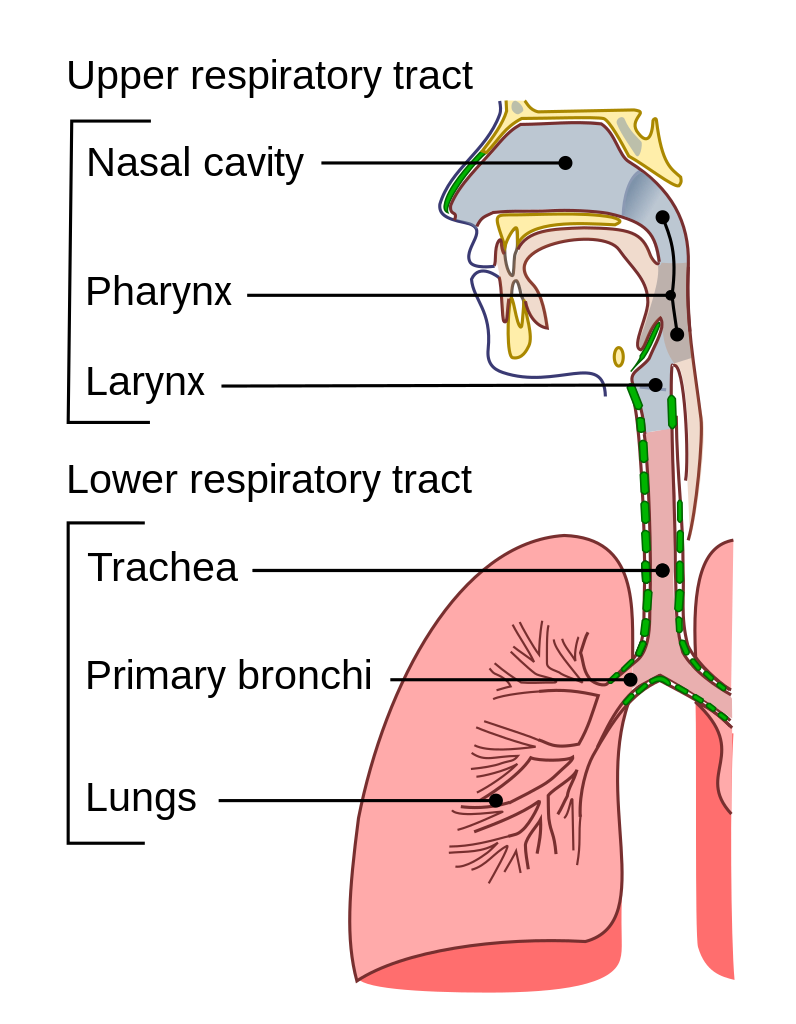
A strong systemic immune response from intramuscular vaccination may not necessarily correlate with a strong mucosal immune response (mediated primarily by IgA) in the upper airways (1). Therefore, vaccinated individuals can still exhibit positive results in SARS-CoV-2 PCR tests—which typically use nasal or nasopharyngeal swabs for sample collection-—because the virus may be present at detectable levels in the upper respiratory tract. Earlier studies have shown that the incidence of breakthrough infections in fully vaccinated individuals is extremely low (2,3). However, a recent study from the University of Wisconsin–Madison and collaborators showed that fully vaccinated individuals infected with the Delta variant, which is more infectious than the original virus, “shed” similar amounts of the virus as unvaccinated individuals (4).
A previous blog post discussed the difference between systemic and mucosal immunity, and this topic has been extensively reviewed (5,6). The blog post proposed one possible solution to stopping the virus at the source of infection: intranasal SARS-CoV-2 vaccines. A Science perspective published in July 2021 notes, “it seems surprising that only seven of the nearly one hundred SARS-CoV-2 vaccines currently in clinical trials are delivered intranasally” (7). The authors review current research on intranasal vaccines and make a case for their advantages over intramuscular injection, both for fighting SARS-CoV-2 infection at the source and for reducing viral shedding, which can spread the disease.
Intranasal Vaccine Development
Intranasal vaccines offer other advantages over current mRNA vaccines. They are easier to administer and may be more accepted than intramuscular injections, do not require extended ultracold storage, and are especially suited for mass distribution in countries where a high proportion of the population remains unvaccinated. However, intranasal vaccine development does face some challenges.
Current lipid-encapsulated mRNA vaccines are not formulated for intranasal administration (7). Any intranasal vaccine must overcome the barrier posed by nasal mucus, in order for the antigen to be effectively delivered to nasal epithelial cells. Most of the intranasal SARS-CoV-2 vaccines in clinical trials at present are either adenoviral vector vaccines or live-attenuated virus vaccines. Their safety and efficacy have yet to be conclusively demonstrated. Results from selected preclinical studies illustrating different approaches to intranasal vaccine development are summarized here.
- An et al. used a novel approach using lyophilized SARS-CoV-2 Spike protein and an agonist of the cyclic GMP-AMP synthase and the stimulator of interferon gene (STING) pathway as an adjuvant (8). The STING agonist was encapsulated in liposomal nanoparticles to increase the efficiency of vaccine delivery. The vaccine induced both mucosal and systemic immunity in mice when delivered intranasally. The vaccine is being commercialized by AuraVax Therapeutics.
- The chimpanzee adenoviral vector vaccine, ChAdOx1 nCoV-19, was developed by the University of Oxford and AstraZeneca, and is currently approved for intramuscular injection. In a new study, the vaccine was administered intranasally to macaques and hamsters, followed by a challenge with a mutant SARS-CoV-2 strain (9). In both animals, viral loads were decreased in nasal swabs compared to control groups, and reduced or eliminated in lower respiratory tract tissue.
- In a different viral vector approach, Ohtsuka et al. used a replication-incompetent human parainfluenza virus type 2 (hPIV2) vector expressing a prefusion-stabilized Spike protein (S-2PM), as well as several other mutants (10). Intranasal vaccination of mice with S-2PM-based vaccine induced the production of both IgG and IgA neutralizing antibodies. Single-dose intranasal vaccination of hamsters offered complete protection against SARS-CoV-2 in the lungs, and a booster 2 weeks before viral challenge resulted in virtually complete protection in the nasal passages. Similar results were obtained by Park et al. using a Newcastle disease virus (NDV) vector expressing Spike protein (11).
- A study by Meissa Vaccines and collaborators used a live-attenuated recombinant human respiratory syncytial virus (RSV) expressing a chimeric SARS-CoV-2 Spike protein (12). A single intranasal dose in African green monkeys generated both mucosal and systemic immunity against homologous SARS-CoV-2 as well as Alpha and Beta variants. Further, the vaccine reduced viral shedding in the nose by more than 200-fold after viral challenge. The vaccine is currently in phase 1 clinical trials.
The current state of intranasal vaccine development efforts has been reviewed comprehensively by Chavda et al. (13). More information about ongoing intranasal SARS-CoV-2 clinical trials is available at clinicaltrials.gov.
What’s Next?
Preliminary clinical trial data from CanSino Biologics’ intranasal adenovirus-vectored vaccine showed encouraging results. Two intranasal doses elicited a neutralizing antibody response similar to that of one intramuscular dose (14). Bharat Biotech (India) also has an adenoviral vector intranasal vaccine in clinical trials. As other clinical trials progress, we should have a more detailed picture of intranasal vaccine effectiveness and safety, especially in reducing the incidence of breakthrough infections. Ultimately, it may be possible that intramuscular injection followed by an intranasal booster produces the most potent response (7). Meanwhile, a combination of current vaccination protocols and mitigation efforts, such as mask use, remain the best defense.
Learn about tools to study SARS-CoV-2 with our SARS-CoV-2 Drug Discovery and Vaccine Development page.
References
- Wisnewski, A.V. et al. (2021) Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 16(6), e0249499.
- Thompson, M.G. et al. (2021) Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—Eight U.S. locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 70(13), 495–500.
- Keehner, J. et al. (2021) SARS-CoV-2 infection after vaccination in health care workers in California. N. Engl. J. Med. 384, 18.
- Riemersma, K.K. et al. (2021) Shedding of infectious SARS-CoV-2 despite vaccination. [medRxiv preprint] https://doi.org/10.1101/2021.07.31.21261387
- Russell, M.W. et al. (2020) Mucosal immunity in COVID-19: A neglected but critical aspect of SARS-CoV-2 infection. Front. Immunol. 11, 611337.
- Ratcliffe, N.A. (2021) Nasal therapy—The missing link in optimizing strategies to improve prevention and treatment of COVID-19. PLoS Pathog. 17(11), e1010079.
- Lund, F.E. and Randall, T.D. (2021) Scent of a vaccine: Intranasal vaccination should block SARS-CoV-2 transmission at the source. Science 373(6553), 397–399.
- An, X. et al. (2021) Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience 24, 103037.
- van Doremalen, N. et al. (2021) Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 13, eabh0755.
- Ohtsuka, J. et al. (2021) Non-propagative human parainfluenza virus type 2 nasal vaccine robustly protects the upper and lower airways against SARS-CoV-2. iScience, 2021, 103379.
- Park, J.-G. et al. (2021) Immunogenicity and protective efficacy of an intranasal live-attenuated vaccine against SARS-CoV-2. iScience 24, 102941.
- Tioni, M.F. (2021) One mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. [bioRxiv preprint] https://doi.org/10.1101/2021.07.16.452733
- Chavda, V.P. et al. (2021) Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management. Drug Discov. 26(11), 2619–2636.
- Wu, S. et al. (2021) Safety, tolerability, and immunogenicity of an aerosolized adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 21, 1654–1664.
Latest posts by Ken Doyle (see all)
- Will Artificial Intelligence (AI) Transform the Future of Life Science Research? - February 1, 2024
- RAF Inhibitors: Quantifying Drug-Target Occupancy at Active RAS-RAF Complexes in Live Cells - September 5, 2023
- Synthetic Biology: Minimal Cell, Maximal Opportunity - July 25, 2023
