NAD is a pyridine nucleotide. It provides the oxidation and reduction power for generation of ATP by mitochondria. For many years it was believed that the primary function of NAD/NADH in cells was to harness and transfer energy from glucose, fatty and amino acids through pathways like glycolysis, beta-oxidation and the citric acid cycle.
However NAD also is recognized as an important cell signaling molecule and substrate. The many regulatory pathways now known to use NAD+ in signaling include multiple aspects of cellular homeostasis, energy metabolism, lifespan regulation, apoptosis, DNA repair and telomere maintenance.
This resurrection of NAD importance is due in no small part to the discovery of NAD-using enzymes, especially the sirtuins.
NAD/NADH Backstory
In both prokaryotes and eukaryotes, NAD is synthesized by two pathways. In the de novo pathway, NAD is synthesized from tryptophan, but also is created in the salvage pathway from recycling of degraded NAD products such as nicotinamide. In yeast, a model system for eukaryotes, the de novo and salvage pathways for NAD synthesis play redundant but essential roles in cell growth (1).
NAD is important in numerous biological pathways, including energy metabolism regulation, DNA transcription and repair. NAD also functions as a substrate for enzymes such as DNA ligases, NAD-dependent oxidoreductases, poly-ADP-ribose polymerase (PARP), and more recently the Sir2p family of NAD-dependent deacetylases (1).
NADH, the reduced form of NAD, is a substrate for NADH dehydrogenase, part of the mitochondrial respiratory chain that transfers electrons to coenzyme Q, to generate NAD.
NAD/NADH has been shown to regulate the binding of corepressor CtBP to transcriptional repressors. CtBP is involved in transcription pathways for cell growth and differentiation and transformation. Studies have shown NADH to be 2–3 orders more effective than NAD at enhancing this CtBP binding to repressors.
A Role in Disease
Several diseases have been tied, directly or indirectly, to changes in NAD level or NAD/NADH balance. NAD/NADH levels regulate corepressor CtBP activity, and have thus been shown to have a role in carcinogenesis (1).
PARP1 has been implicated in mechanisms underlying type 1 diabetes. PARP1 uses NAD as a substrate and plays a role in DNA-base excision repair, signaling due to DNA damage and regulation of transcription and proteosomal function. Overactivation of PARP1 leads to NAD depletion and cell death by necrosis.
NAD depletion by PARP1 also is believed to play a role in diabetic endothelial dysfunction.
Thus from this rudimentary examination of NAD’s role in cells, one can conclude that if the availability of NAD to cells is somehow disrupted, cellular function would be seriously and negatively impacted. What is bad for the cell is quite definitely bad for the organism.
A Role in Aging
Diabetes and related vascular dysfunctions and metabolic diseases are often referred to as age-related diseases. Calorie restriction (CR) research, which examines how eating less may improve longevity, points to the seemingly strong connection of NAD to aging. Sirtuins (SIRT in mammalian research, Sir2 in the yeast research) are NAD+-dependent deacetylases and ADP-ribosylases that have been shown to play a role in the regulation of stress response, gene transcription, cellular metabolism and longevity.
Sirtuins are proteins that are known to be evolutionarily conserved, thus yeast research poses a good model for what may be happening in mammalian systems. Researchers propose that though the coupling by sirtuins of cleavage of NAD with the modification of target proteins, sirtuins could be a molecular link “relaying the cellular energy state to the machinery of life span regulation” (2).
Calorie restriction is the most effective means known to extend lifespan in a variety of organisms, including mammals. Moderate CR can be caused in yeast by reducing the glucose concentration in culture media (from 2% to 0.5%). Lifespan increases caused by low-glucose consumption has been shown to require both NAD+ and Sir2 (2).
NAD, from pathway to powerhouse. No one would have doubted that their role in respiration and mitochondrial function was important, but learning about their function in CR and longevity garners new respect for this old molecule.
Interested in detecting NAD/NADH? Learn about the simple, sensitive, high-throughput NAD/NADH-Glo™ Assay!
References
- Lin, S.-J. and Guarente, L. (2003) Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr. Opin. Cell Biol. 15, 241-6.
- Lu, S.-P. and Lin, S.-J. (2010) Regulation of Yeast Sirtuins by NAD+ Metabolism and Calorie Restriction. Biochim Biophys Acta. 1804, 1567-75.

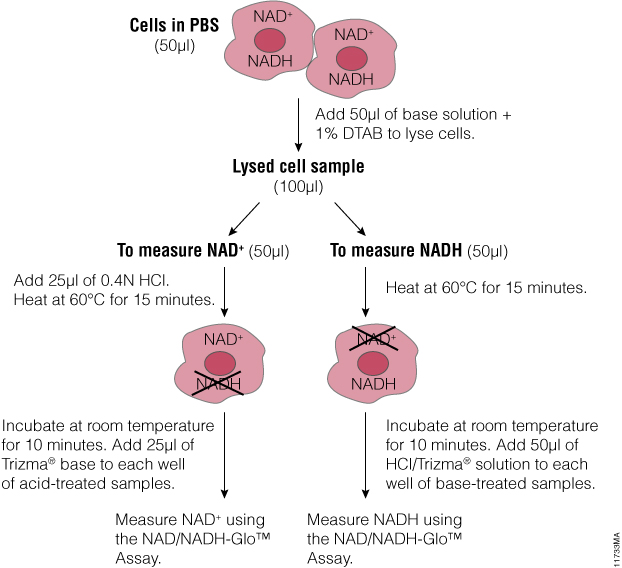
Can you elaborate on the composition of the 50ul “base solution” + 1%DTAB in the first step of the procedure schematic?
The composition of the solution is provided in TM399 (Section 5, page 13): base solution with 1% DTAB (bicarbonate base buffer with 1% DTAB or 0.2N NaOH with 1% DTAB
Here is the link to the technical manual: https://www.promega.com/-/media/files/resources/protocols/technical-manuals/101/nad-nadh-glo-assay-protocol.pdf?sc_lang=en?la=en if you need additional information.
Please let us know if we can be of further assistance.