
In the nine months since the first cases of COVID-19 were noticed in Wuhan, China, the virus has spread around the globe and infected over 22 million people. As with all emerging infectious diseases, we often find ourselves with more questions than answers. However, through the tireless work of researchers, doctors and public health officials worldwide, we have learned a lot about the virus, how it spreads and how to contain it.
SARS-CoV-2: The Virus
On December 31, 2019, Chinese health authorities reported an outbreak of lower respiratory infections in Wuhan City, China. All confirmed cases at that point indicated that the outbreak originated at a market selling live poultry and seafood. Those infected first experience fever and severe cough, along with pneumonia and lung lesions. The virus was quickly identified as a novel coronavirus of unknown zoonotic origin.
Coronaviruses get their name from the halo of fringe surrounding their virions, which resemble the solar corona. Notable examples include Severe Acute Respiratory Syndrome (SARS-CoV), which infected over 8,000 individuals and caused 774 deaths in 2003, and Middle East Respiratory Syndrome (MERS-CoV), which has infected almost 2,500 and caused 858 deaths since 2012.
Coronaviruses are notable for infecting animals such as cows, chickens and pigs, in addition to humans. Disease-causing coronaviruses often originate in non-human animals and later leap human populations. SARS-CoV, for example, was eventually traced to civets, which were an intermediate step for the virus originating in a species of bats. The zoonotic origins of SARS-CoV-2 are still unconfirmed, but bats, civets and pangolins are all under investigation.
Previous reports referred to the virus as “Wuhan Coronavirus,” “Novel Coronavirus” and “2019-nCoV.” The virus was officially named “SARS-CoV-2” by the International Committee on Taxonomy of Viruses on February 11. The disease caused by SARS-CoV-2 is called “COVID-19.”
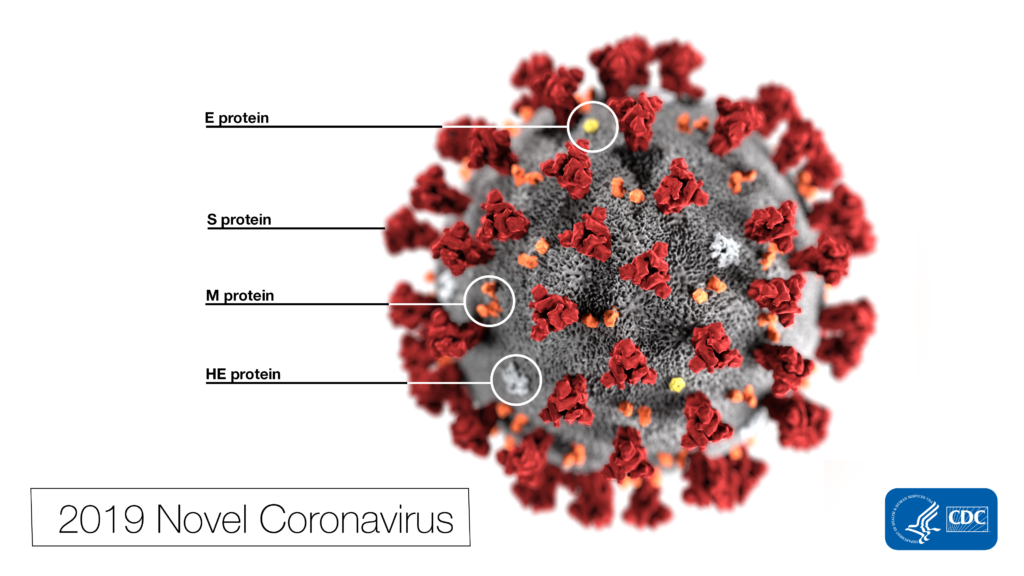
The genome of the SARS-CoV-2 virus is approximately 29,903 bases of RNA which encode for 29 proteins. A detailed description of all know SARS-CoV-2 proteins is available from the New York Times. The structure of the viral spike protein was first published in Science on February 19. The S1 subunit of the Spike protein binds to the human ACE2 receptor with remarkable efficiency, giving the virus access to the interior of host cells. ACE2 is widely distributed in the human body, which could potentially explain some of the non-respiratory symptoms of COVID-19.
COVID-19: The Disease
The World Health Organization classified COVID-19 as a pandemic on March 11, 2020. As of August 21, 2020, 22,789,780 cases have been reported worldwide. The United States leads with over 5.5 million cases, followed by Brazil (3.5 million) and India (2.8 million). There have been 795,575 reported deaths, with the United States again leading with 174,924. A live map of confirmed infections is available from Johns Hopkins University.
The CDC reports that the overall hospitalization rate for COVID-19 in the United States is 144.1 per 100,000 infections. The percentage of deaths in the United states attributed to COVID-19 or related illnesses was 8.1% during Week 32 (August 3-9). Case-fatality ratios have varied widely by country, with the United States at 3.1% but other severely impacted countries as high as 10.9% (Mexico) or as low as 1.7% (Russia).
According to the WHO, the most common symptoms of COVID-19 are fever, dry cough and tiredness. A wide range of other symptoms have been reported with varying frequency, including lost of taste or smell and various digestive issues. Some people who are infected will experience very mild or no symptoms, while many will have an experience similar to a bad cold. Approximately 20% of people who are infected develop severe symptoms including difficulty breathing.
Emerging reports have documented additional non-respiratory symptoms associated with some cases of COVID-19. Cytokine storms been linked to many severe cases of the disease. This autoimmune syndrome involves the massive release of inflammatory cytokines, which often triggers acute respiratory distress syndrome (ARDS). There are also many cases of COVID-19-related myocarditis, which is thought to be caused by direct attack by the virus and cardiac damage from the patient’s immune response.
It has long been established that diabetes was associated with increased risk of severe COVID-19, but growing evidence suggests there is a link between the disease and newly onset diabetes as well. The CoviDIAB Project is a registry of patient data for researchers studying the link between COVID-19 and type 1 or type 2 diabetes. Finally, several studies have found elevated risk of ischemic stroke in COVID-19 patients compared with influenza patients, as well as cases of encephalitis, encephalopathy, psychosis and dementia-like cognitive issues. Research on the causes of these pathologies is ongoing.
Throughout the pandemic, elderly individuals have seen the highest rates of severe disease and fatality. For several months, the general consensus was that young children had very low susceptibility. However, more recent reports have indicated that children are both susceptible and likely to transmit the infection. For example, a CDC report on an overnight camp in Georgia that at least 44% of attendees contracted the disease while at the camp (Only 58% of attendees reported test results, but 76% of those were positive). At this time, children are assumed to be at similar risk of infection and transmission as adults.
Testing
Most of the first COVID-19 testing uses PCR-based methods. CDC offers protocols for qPCR-based detection, and the FDA approved several commercially available kits for Emergency Use (EUA). These tests typically start with an upper respiratory nasopharyngeal swab. The testing lab will extract RNA from the sample and probe for RNA from the SARS-CoV-2 virus.
Antibody tests are increasingly receiving attention as another important tool for understanding the spread of the virus. These tests detect the presence of anti-SARS-CoV-2 antibodies in blood samples. The presence of the antibodies indicates that the individual has been previously infected with the virus. Widespread antibody testing could potentially help public health officials build a better picture of what percentage of the population has been infected, and thus help predict how the virus is likely to spread.
The first saliva test for COVID-19 was granted EUA by the FDA on August 15, 2020. Researchers at the Yale School of Public Health developed the test in collaboration with the NBA. The team plans to make its protocols open-access for testing labs around the country.
Treatments and Vaccines
At this time, the only drug to receive EUA from the FDA is Remdesivir, an antiviral developed by Gilead Sciences. Remdesivir was designed to target hepatitis C virus (HCV) and respiratory syncytial virus (RSV) and was later tested against Ebola. Remdesivir is an adenine analog, which acts by disrupting the viral RNA-dependent RNA polymerase.
Hydroxychloroquine has received a lot of attention as a potential treatment for COVID-19. This drug, approved for use in treating malaria, lupus and rheumatoid arthritis, received EUA on April 27, 2020. This authorization was later revoked on June 15 after further study suggested that the drug was unlikely to be effective against COVID-19. The FDA went even further on July 1 when they specifically cautioned against its use for COVID-19 due to elevated risk of complications with heart rhythms, kidneys and liver.
As of August 20, 44 vaccine candidates are at least in Phase 1 clinical trials around the world. Two vaccines have been approved for limited use. The Chinese military approved a vaccine developed by CanSino Biologics on June 25 as a “specially needed drug,” before the vaccine had entered Phase 3 trials. The second approved vaccine was developed by the Gamaleya Research Institute in Russia. This vaccine was also approved before entering Phase 3 trials. Other promising candidates include a messenger RNA vaccine developed by Moderna. Moderna has collaborated with the National Institutes of Health (NIH) to complete Phase 1 and Phase 2 trials, and Phase 3 began on July 27, 2020.
Staying Safe
The virus is most often spread through the air when two people are in close contact, so doctors and public health officials strongly recommend limiting the size of gatherings, maintaining physical distancing and avoiding indoor gatherings with possible. Many areas still have restrictions on business occupancy and public events, and we recommend following all local guidelines.
Face masks or other coverings have been shown to limit transmission. Some areas require masks to be worn in public spaces. It is still a good practice to wear a face covering even if it is not required.
Above all, it’s crucial to maintain good hygiene by washing your hands regularly and covering your mouth and nose when coughing and sneezing.
Promega is prepared to support scientists in their work towards understanding SARS-CoV-2 and developing drugs to treat the infection. Click here for more information.
For more information on SARS-CoV-2:
CDC Guidelines and Information
Related Posts
Latest posts by Jordan Villanueva (see all)
- Tackling Undrugged Proteins with the Promega Academic Access Program - March 4, 2025
- Academic Access to Cutting-Edge Tools Fuels Macular Degeneration Discovery - December 3, 2024
- Novel Promega Enzyme Tackles Biggest Challenge in DNA Forensics - November 7, 2024

great article but do you have any study on the vaccination of the novel coronavirus??
Hello,
Thanks for your interest in our article. We are continually updating it as new information becomes available. We are collecting information from the research community and supporting their efforts, but we are not conducting any of the research into this virus ourselves.